Analytical Development
Analytical Development Companies (58)
Analytical Development News
-
News Skyepharma selects Veratrak platform to ensure secure data storing and sharing
Using Veratrak's platform, the CDMO will have full oversight of data, including an audit trail of data and document processes -
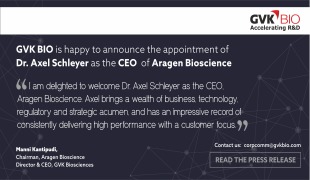
News Vectura to provide preclinical support for Incannex's inhaled brain injury treatment
IHL-216A may make sports safer and reduce the morbidity and mortality rates of people suffering serious head traumas -
News MedPharm expands topical and transdermal delivery services
A new location in Raleigh-Durham, North Carolina, close to the CDMO's Center of Excellence, will double its current footprint -
News CURE Pharmaceutical gains DEA go-ahead to research psychedelics compounds
Company will use extension of Schedule I licence to start development of psychedelics-based pipeline to treat mental health disorders
Analytical Development Products (52)
-
Product Biosafety Testing & Analytical Development Services
Choosing the right partner for analytical and biosafety testing is critical in the race to approval. Our BioReliance® Contract Testing Services offer exceptional, risk-mitigating solutions with technical and regulatory expertise, to help bring life-changing drugs to market.
-
Product Analytical Development
With top expertise in protein analytics, KBI has successfully completed over 3300 analytical projects for more than 300 customers and more than 130 distinct molecules.
Our experience includes antibodies (IgG1, IgG4, IgM, FAb, ADC, Fc fusion), enzymes, cytokines, growth factors, highly glycosylat...
-
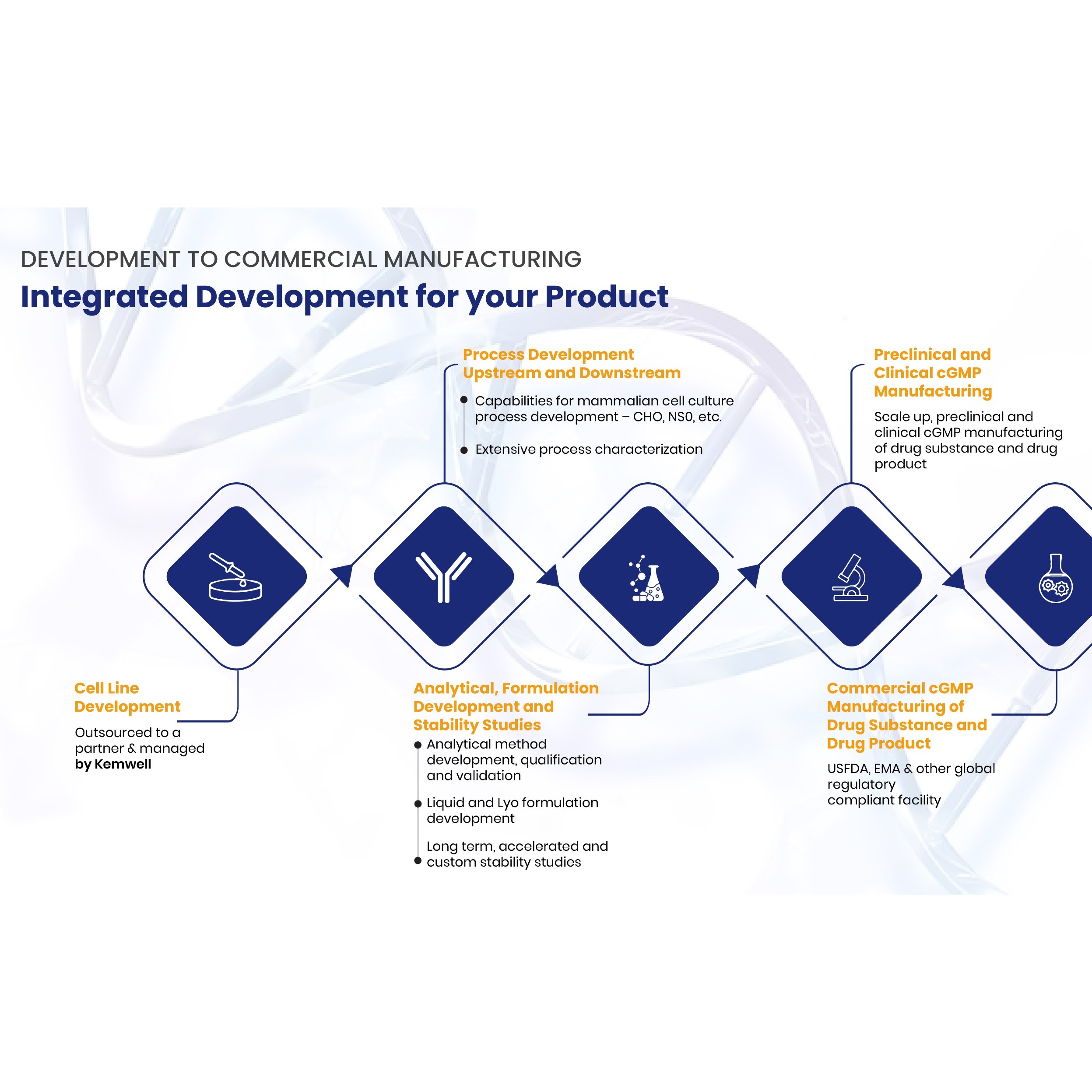
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product GMP and CMC Laboratory Services
Laboratory services according to GMP and CMC: We provide regulatory-driven, phase-appropriate laboratory services, supporting CMC programs from preformulation to formulation to product release. Among our capabilities are centres of excellence for method development and validation, analysis, stability s...
-
Product Analytical Services
Solvias provides cGMP-compliant contract analytical services to help you ultimately provide safer products to consumers. We provide a comprehensive range of analytical services to the pharmaceutical, biotech, medical devices and cosmetics industry with similar regulatory requirements for raw materia...
-
Product Analytical Services
With a team of experienced scientists and a robust toolbox of analytical techniques, Experic can support the life cycle of your oral solid dose and/or inhalation pharmaceutical products. Our laboratory staff provides comprehensive analytical solutions to support the entire spectrum of development and manuf...
-
Product GMP Analytical Testing
Singota solutions offers wide range of services which includes GMP analytical testing.
Studies & Methodologies: • Material Identity • In Process • Product Release • Product Specific Assays • Compendial • Microbial (Endotoxin, Bioburden, Sterility) • CoA Generation • ICH Stability (All ICH...
-
Product Analytical Services & EU Batch Certification
Method Development and validation
Stability Studies
European testing & batch certification

-
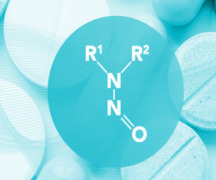
Product Nitrosamine Analysis
In response to nitrosamines present in pharmaceutical products, the European Medical Agency (EMA) and the US Food and Drug Administration (US FDA) have published requirements and limits related to nitrosamine contaminants. Therefore Brightlabs has developed and validated multiple methods to determine low l...
-
Product Inhalation Test Services
We are the world’s leading provider of orally inhaled and nasal drug product design and development services. We enable the seamless translation of pre-clinical development through to clinical manufacture of OINDPs. We do this through our unique processing technologies and formulation development tools....
-
Product A CDMO PARTNER FOR LIFE
FUJIFILM Diosynth Biotechnologies is an industry-leading cGMP Contract Development and Manufacturing Organization (CDMO) supporting the biopharmaceutical industry in the development and production of biologics, vaccines and cell and gene therapies. Our focus is to combine technical leadership in process d...
-
Product Analytical Development & QC
Based on an analytical continuum our testing experts provide solutions all along the value chain from early discovery to clinical trials including method development, validation, quality control, stability studies of the main physicochemical and microbiological testing for innovative and generic products.
-
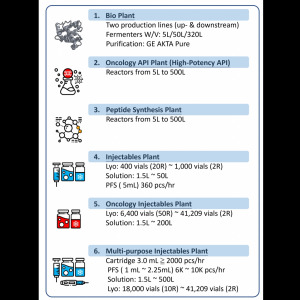
Product GBC Production Plant site Video
Genovior Biotechnology Corporation facility capabilities & current ongoing production lines
-
Product Oral Dosage Forms
We have extensive experience and know-how in the development of oral dosage forms containing spray dried powders. Tablets and capsules are the most common dry powder dosage form. However, other dosage forms such as sachets (bulk powders), vials (for reconstitution) have also be developed by the Upperton team.
-
Product ADAPTEK® TECHNOLOGY
ADAPTEK® TECHNOLOGY is the general name for our 4 international patents and expertise in nanotechnology and controlled release systems.This is a transversal technology that allows to develop customized biopolymeric nanohydrogels, allowing the load with different API (drugs, vitamins, growth factors, etc.)....
-
Product Analytical Services
Pharmaffiliates is providing all the Analytical Services (i.e. Method Development, Method Validation & Transfer, Stability Studies, Contract Analysis, etc)
-
Product Analytical Development
BioDuro-Sundia’s Analytical Testing team offers high quality analytical services including method development and validation, qualification of reference standards, testing and release studies, stability studies, and CMC dossier preparation services. ...
-
Product PILOT BATCHES AND REGISTRATION BATCHES CONTRACT MANUFACTURING
TECNALIA, experts in Pharmaceutical Development, Scale-up & Pilot Batches Manufacturing, Clinical Trials and Contract ManufacturingPilot
Batches and Registration Batches /
• Manufacture in compliance with the principles and guidelines of GMP for medicinal products.
• Transfer o...
-
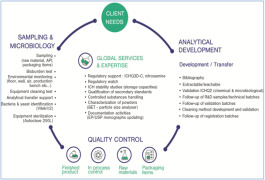
Product Analytical Development and expertise
Our analytical experienced team support the pharmaceutical development during every phase of the development process. Analytical development transfer and validation, characterization capabilities, stability analysis, ICH Q3D (residuals solvent risk assesment), ICHQ3C (elemental impurities risk assesment...
Upcoming Events
-
CPHI Japan 2024
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
17 Apr 2024 – 19 Apr 2024 -
CPHI North America 2024
Pennsylvania Convention Center, Philadelphia
07 May 2024 - 09 May 2024 -
CPHI & PMEC China 2024
Shanghai New International Expo Center
19 - 21 June 2024
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





























































.jpg)





























































.png)


.png)



.jpg)