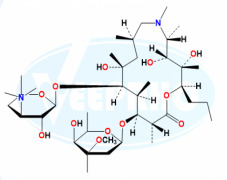
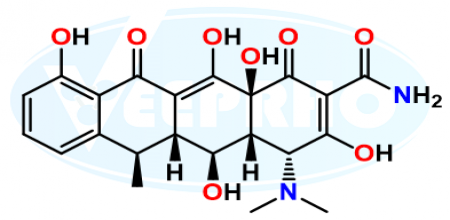
Doxycycline EP Impurity C
Product Description
Veeprho Pharmaceuticals s.r.o.

-
CZ
-
2019On CPHI since
-
2Certificates
-
100 - 249Employees
Company types
Categories
Specifications
Veeprho Pharmaceuticals s.r.o.

-
CZ
-
2019On CPHI since
-
2Certificates
-
100 - 249Employees
Company types
More Products from Veeprho Pharmaceuticals s.r.o. (2)
-
Product Azithromycin EP Impurity O
Name:Azithromycin EP Impurity OCatalogue No.VL1860010CAS No.763924-54-5Molecular FormulaC39H74N2O12Molecular Weight763.01StatusIn-stockIUPAC Name(2-desethyl-2-propylazithromycin) -
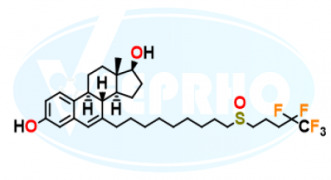
Product Fulvestrant EP Impurity E
Fulvestrant EP Impurity E / Delta 6,7 Fulvestrant Impurity
Catalogue No: VL234005
Molecular Formula: C32H45F5O3S
Molecular Weight: 604.75
Status: In-stock
IUPAC Name: 7-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfin...
Veeprho Pharmaceuticals s.r.o. resources (1)
-
Brochure Company Brochure
Company brochure of our sister company Veeprho Laboratories Pvt. Ltd. where all our products are manufactured.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance