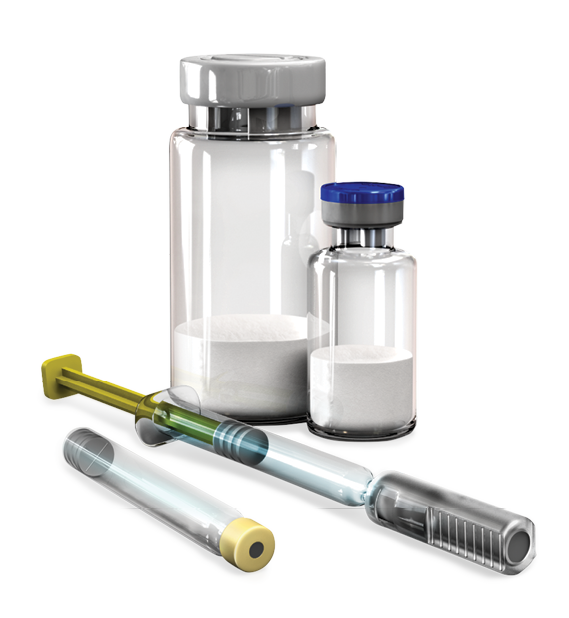
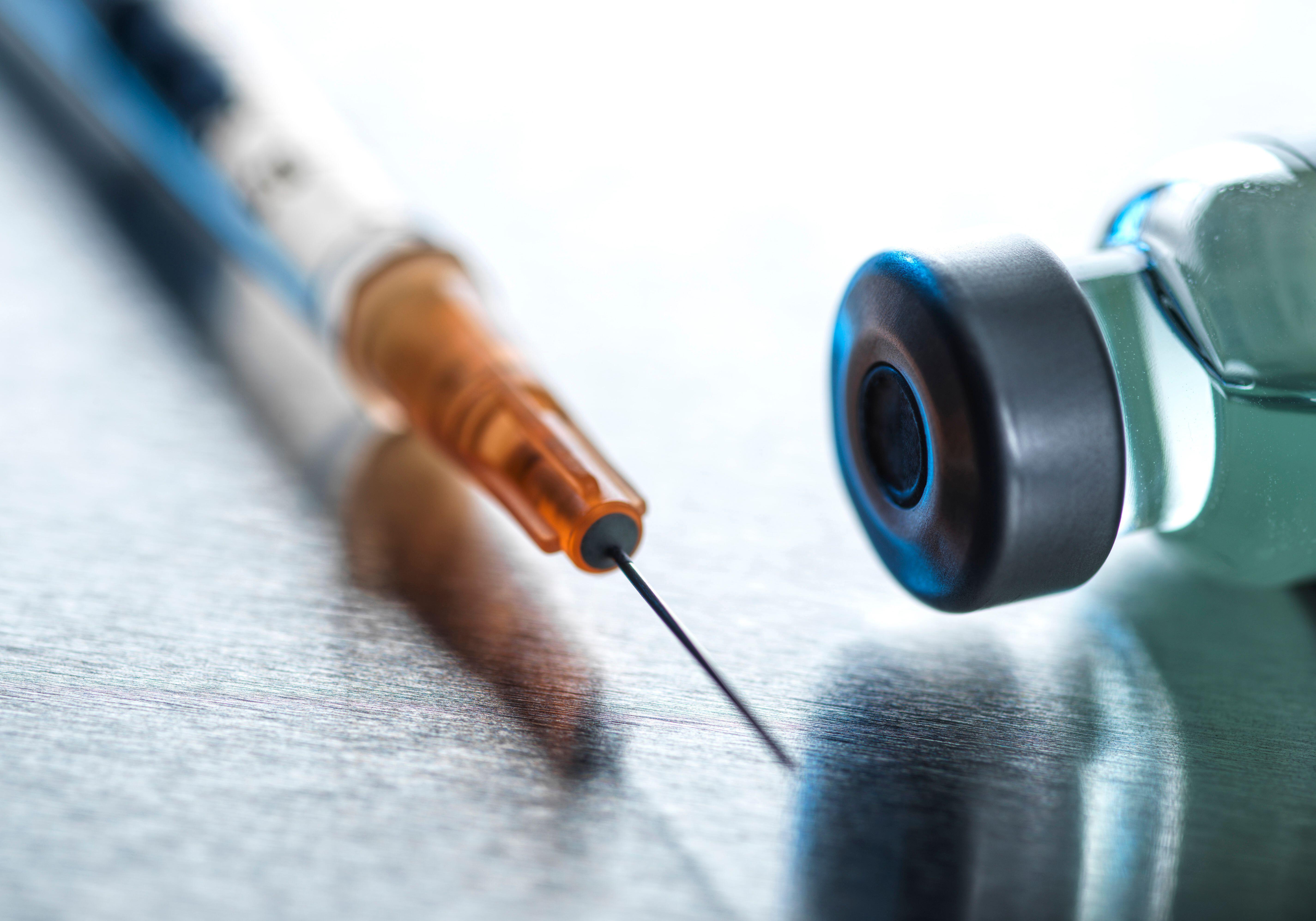
Antibody-Drug Conjugates

Product Description
Baxter Healthcare Corporation

-
US
-
2015On CPHI since
-
2Certificates
-
5000+Employees

Categories
Baxter Healthcare Corporation

-
US
-
2015On CPHI since
-
2Certificates
-
5000+Employees

More Products from Baxter Healthcare Corporation (9)
-
Product Powder Filled Vials
Baxter (BioPharma Solutions) offers a wide range of complex sterile manufacturing products which includes powder filled vials. It is a type of cytotoxics. It offers filling of powder filled vials at our highly experienced Halle/Westfalen, Germany facility. In addition to continuous monitoring of temperatur... -
Product Diluents for Reconstitution
Baxter (BioPharma Solutions) offers a wide range of traditional sterile manufacturing products which includes diluents for reconstitution. It offer both customer and patient benefits. When compared to vials, they can enhance and improve patient safety such as less steps for reconstitution; reduced contamin... -
Product Cytotoxics
Baxter (BioPharma Solutions) offers a wide range of complex sterile manufacturing products which includes cytotoxics. It is the most highly sophisticated and sensitive drugs to handle and produce. The barrier technology ensures your cytotoxic product is manufactured under precisely controlled temperature, ... -
Product Highly Potent Compounds
Baxter (BioPharma Solutions) offers a wide range of complex sterile manufacturing products which includes highly potent compounds. It presents many challenges due to the complex handling required for toxic substances. Utilizing cRABs (closed restricted access barrier system) for commercial manufacturing al... -
Product Biologics
Baxter (BioPharma Solutions) offers a wide range of complex sterile manufacturing products which includes biologics. It offers innovative filling line technologies that are specifically designed to protect your biologic's integrity and optimize yield of costly API. This manufacturing process optimization h... -
Product Sterile Crystallization
Baxter (BioPharma Solutions) offers a wide range of complex sterile manufacturing products which includes sterile crystallization. It is a type of cytotoxics. It offers sterile crystallization of cytotoxic active pharmaceutical ingredients in a closed system. Sizes and options: poly ethylene bags (1 - 5 kg... -
Product Prefilled Syringes
Baxter (BioPharma Solutions) offers a wide range of traditional sterile manufacturing products which includes prefilled syringes. It offers expanded small-scale through high-volume sterile manufacturing. It can increase production potential and help ensure on-time delivery to meet product demand. By provid... -
Product Lyophilized Vials
Baxter (BioPharma Solutions) offers a wide range of complex sterile manufacturing/traditional sterile manufacturing products which includes lyophilized vials. The process of freeze drying can achieve product stability, and improved shelf-life. It offers individual filling suites with lyophilization capacit... -
Product Liquid Vials
Baxter (BioPharma Solutions) offers a wide range of traditional sterile manufacturing products which includes liquid vials. It can helps clients to meet developmental, clinical, and commercial liquid vial manufacturing needs. High-volume filling and multiple, dedicated vial-filling suites allow to expand p...
Baxter Healthcare Corporation resources (12)
-
Sponsored Content CPHI Podcast Series: Key Considerations in Selecting the Right CMO Partner
In this month's episode we hear from Jayna Blake, Senior Project Manager for Technical Programs at Baxter BioPharma Solutions, on key considerations for successful CMO selection. -
Brochure A Global Leader in Sterile Contract Manufacturing
Our processes and systems are tailored to meet your parenteral manufacturing needs. We offer conventional sterile dosage forms, including prefilled syringes, liquid vials, lyophilized vials and cartridges as well as cost-saving diluents for reconstitution. -
News Baxter announces $100M investment in biopharma solutions at German sterile fill/finish manufacturing facility
Investment will add a syringe filling line, additional liquid and lyophilized (freeze dried) vial capacity and expand manufacturing footprint -
Brochure Complex Sterile Manufacturing: More Than 60 Years of Oncology Experience
Parenteral manufacturing can be a complicated process. Cytotoxics, antibody-drug conjugates (ADCs), highly potent compounds, biologics and lyophilized products present many challenges and require specialized understanding and expertise. -
Sponsored Content Audits in the time of COVID-19 – Implications for a CMO
Travel restrictions imposed by the pandemic have limited the ability of pharmaceutical companies and health authority personnel to travel and conduct in-person inspections of contract manufacturing organizations (CMOs).
Due to these limitations, both health authorities and CMOs must adapt and move to new processes for conducting audits. This can be done by leveraging innovation and technology solutions.
-
Brochure Maximize Your Molecule's Full Potential
We know the high-stakes challenges you face in today's complex parenteral marketplace - and how the work we do is vital to the patients you serve. That's why we work closely with you at every step to help you achieve your molecule's full potential and your commercialization objectives. -
News CPHI releases Pharma Trends 2022 report
CPHI has launched its forward-looking pharma trend report for 2022 and it is now available to read on CPHI Online. -
Whitepaper Seasonal Vaccine Manufacturing
The production of seasonal vaccines, such as those for influenza, presents unique challenges to manufacturers due to the necessary time constraints resulting from annual strain selection. The limited-time span from strain selection to distribution requires most vaccine producers to rely on outsourcing partners to ensure timely production and supply. While the right facility and technical expertise are important, vaccine developers should also focus on securing a culture of speed and execution, shared expectations, and continuous improvement. This whitepaper from Baxter’s contract manufacturing business, BioPharma Solutions examines three key areas to evaluate when selecting an outsourcing partner for vaccines production to ensure quality and on-time delivery. -
Whitepaper CPHI Pharma Trends 2022 Report
Via interviews with key industry experts, this highly topical report sponsored by Baxter identifies the major and emerging trends that are sure to be prominent for the pharmaceutical supply chain and outsourcing sector throughout 2022. -
Webinar Rapid Tech Transfer of Vaccine Products
The compressed timelines for technical transfer of COVID-19 projects were unprecedented and offered opportunities to identify and reflect on the unique capabilities required to meet these timelines, as well as the best practices applied to result in the fastest path to GMP production and supply reliability. This webinar is an opportunity for pharmaceutical companies to better understand how the selection of the right CMO is key not only save cost, but also time. Join us to learn more about why the following criteria are so important when selecting a CMO: Responsive and Expansive Supplier Networks Global Manufacturing and Regulatory Experience Collaborative Decision Making Experience and Agility Scientific Expertise -
Webinar Common Stumbling Blocks When Outsourcing an Injectable Project to a CMO & How to Avoid Them
With the recent growth in demand for injectables (due to the COVID-19 pandemic and an increased reliance on biologics to treat chronic diseases) and a tightening of the capacity available, many biotech and pharma companies are having to outsource the manufacturing of their drug products. Yet many have limited-to-no experience working with a CMO. They often do not know what to look for in a vendor or when is the right time to engage with them. They tend to reach out too early before they have the critical information necessary for a CMO to properly define the scope of the project, which can waste valuable time. This webinar is meant to provide Best Practices on what pharma clients need to have established prior to requesting a proposal from a CMO and how to have an effective kick off meeting to ensure a streamlined transfer. -
Podcast CPHI Podcast Series: Key Considerations in Selecting the Right CMO Partner
In this episode we hear from Jayna Blake, Senior Project Manager for Technical Programs at Baxter BioPharma Solutions, on key considerations for successful CMO selection.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance















-file109668.jpg)