Aptar Pharma provides unit-dose nasal spray technology for the treatment of opioid overdose
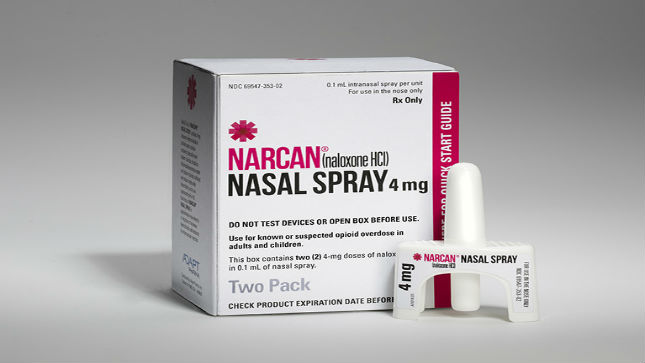
Image - Narcan Adapt Pharma - Courtesy of Adapt Pharma.
The delivery system is for Adept Pharma's Narcan - the first FDA-approved nasally administered, needle-free, ready-to use medication that can stop or reverse the effects of an opioid overdose.
Aptar Pharma has provided the delivery system and regulatory support for Adapt Pharma’s Narcan, which is the first FDA-approved nasally administered, needle-free, ready-to use medication that can stop or reverse the effects of an opioid overdose.
Narcan contains naloxone, which is an opioid antagonist. It is marketed in the US for emergency treatment of known or suspected opioid overdose. “The US is facing an unprecedented opioid overdose epidemic. By making Narcan Nasal Spray available, we hope to increase everyone’s access to emergency treatments across the US,” said Seamus Mulligan, Chairman and CEO of Adapt Pharma.
Aptar Pharma has brought to the partnership its unique experience in nasal drug delivery and regulatory expertise in relation to combination drug-device products successfully supporting Adapt Pharma’s new drug application.
“Aptar Pharma leads the industry in nasal drug delivery expertise. We have collaborated closely with Adapt Pharma to ensure the rapid approval of this potentially life-saving product using our Unit-Dose System (UDS). The regulations and requirements for market approvals are rapidly evolving and customers must seek out trusted partners like Aptar Pharma. We are continually investing in resources and capabilities to support our customers in reaching the market quickly,” said Salim Haffar, President of Aptar Pharma.
Estimated at over $2 billion in 2015, the world market for intra-nasal spray systemic drug delivery has experienced strong growth in the last few years. The list of therapies successfully treated via the nasal route continues to grow from the traditional areas of migraine, osteoporosis and endometriosis, to now include: post-operative pain (ketorolac), cancer pain (fentanyl), osteoporosis, prostate cancer, urinary incontinence, anemia and vaccinations.
Aptar Pharma’s UDS is the preferred liquid spray drug delivery technology platform when dose accuracy and ease of administration are critical. The single-shot UDS system’s primeless feature combined with 360° orientation offer the patient or the caregiver unique ready-to-use convenience.
Related News
-
News Eli Lilly gets ready to launch five new drugs in 2023
Eli Lilly, the American pharmaceutical company (IN, USA) are gearing up for a big year ahead, with hopes to launch five new drugs and capitalise on growing obesity and Alzheimer’s disease markets. -
News Amgen buys Horizon for $27.8 billion in bold step into the rare disease market
Amgen Inc buys pharmaceutical company Horizon Therapeutics in a multibillion-dollar deal, in hopes to capitalise on it's portfolio of drugs in the highly sort after rare disease market. -
News Pharma Supply Chain People Moves
The latest appointments and promotions across the pharmaceutical supply chain. -
News Merck to donate new Ebola vaccine to defend against outbreaks in Uganda
Pharmaceutical giant Merck has announced they will be speeding up the processing of a new vaccine against the latest strain of the Ebola virus, to be donated to a global non-profit organisation for distribution -
News CPHI Podcast Series: Driving innovation with pharmaceutical startups
The latest episode in the CPHI Podcast Series explores how startups are driving innovation by taking high-risk approaches and doing business with greater agility. -
News Greener and efficient processes: Quaternary Ammonium Salts
Quaternary Ammonium Salts play a crucial part in Organic Chemistry processes at many major industries. Discover why.
-
News Biosimilars save patients $11B annually, but barriers to adoption remain in US market
Biosimilars introduce competition into the biologics market, driving down prices and increasing patient access. -
News WHO recommends use of two monoclonal antibody treatments against Ebola
The health body recommended use of treatments by Regeneron and Ridgeback Bio
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




.png)


