Azimed® Tablets
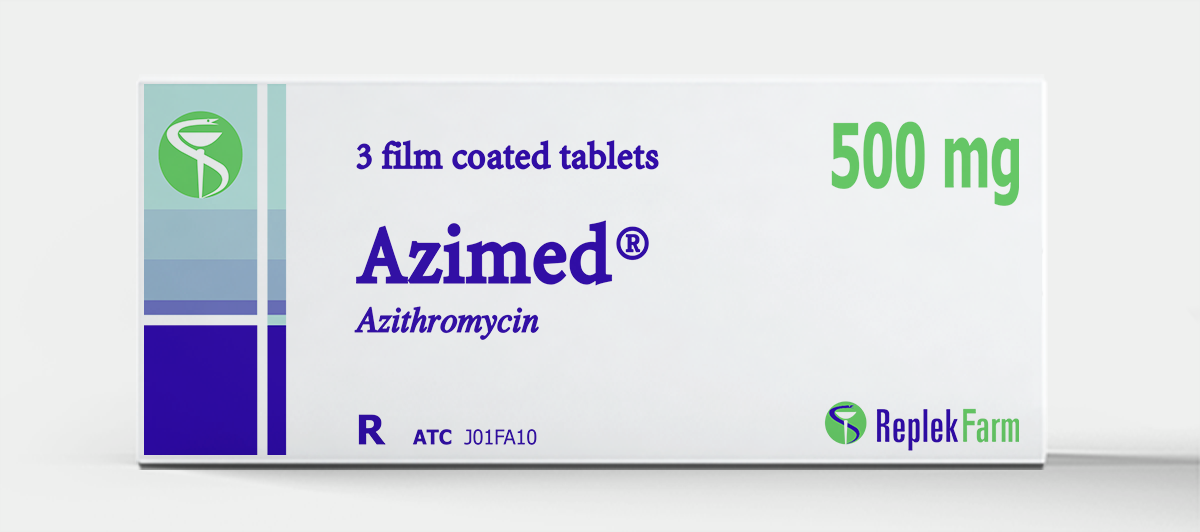
Product Description
Replek Farm

-
MK
-
2022On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
Replek Farm

-
MK
-
2022On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from Replek Farm (4)
-
Product Oralsept® Spray
1 ml solution contains 1.5 mg benzidamine hydrochloride.
Box with a plastic container of 30 ml spray with a dosing pump. -
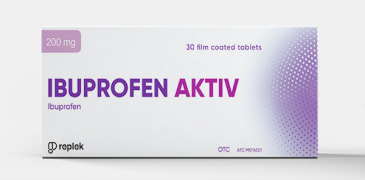
Product Ibuprofen Aktiv® Tablets
1 film-coated tablet contains 200 mg or 400 mg ibuprofen.
Box of 30 film-coated tablets of 200 mg in a blister pack.
Box of 10 film-coated tablets of 400 mg in a blister pack.
Box of 30 film-coated tablets of 400 mg in a blister pack.
-
Product Neuro-Vit®
COMPOSITION
Each film-coated tablet contains:
100 mg thiamin hydrochloride (B1)
200 mg Pyridoxine hydrochloride (B6)
0.2 mg Cyanocobalamin (B12) -
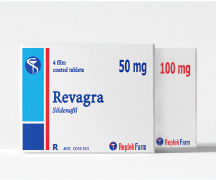
Product Revagra® Tablets
Each film-coated tablet contains 50mg / 100mg sildenafil (in form of sildenafil citrate).
Box with 4 film-coated tablets of 50 mg in blisters.
Box with 4 film-coated tablets of 100 mg in blisters.
Replek Farm resources (1)
-
Video Replek - 20 Years Anniversary
Our concept and our main goal was construction and establishment of a modern factory for production of pharmaceuticals, which was completed in 2001.
Designed, built and equipped in accordance with the latest requirements for good manufacturing practice and the most stringent requirements for environmental protection, today the factory is a modern, operational, high-standardized production capacity.
The facility can produce more than 150 drugs registered in our country and numerous foreign markets, in accordance with the strictest requirements for quality and safety of medicines.
Our future goal is continuous implementation of new technologies in operation, building new capacities, expanding the product range, increasing the number of exporting partners and achieving greater cost-effectiveness.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






-file128286.png)