Baccinex awards Telstar the design and construction of a pharmaceutical aseptic filling process plant in Switzerland
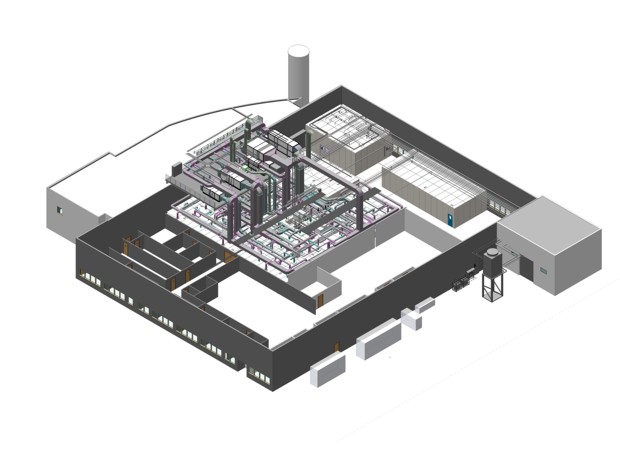
A complete turnkey project.
- The new facility will accommodate an aseptic filling and lyophilization line in an autonomous sterile area, covering the complete process from raw material dispensing to collection of the final product.
- Telstar has taken on the responsibility for the complete critical installation supply, from concept design through basic & detailed engineering to the construction, including the provision of production equipment and utilities, as well as a complete commissioning, qualification and validation service, inclusive of PQ and quality assurance support.
Baccinex has entrusted Telstar with the design and construction of a clean room installation to accommodate an aseptic and lyophilized filling process line, enlarging the existing pharmaceutical plant in the Switzerland region of Jura. The new building, which will be completed in 2019, covers about 1,000 square-meters devoted to the manufacturing of sterile pharmaceutical product batches for clinical trials.
This is a complex turnkey technological project of a sterile production plant equipped with a complete automatic aseptic filling line, which is made up of a vials washing machine followed by a depyrogenation & sterilization tunnel and a vials filling machine operating under laminar flow equipped with a restricted access barrier system (oRABS). Vials are transferred to two freeze-dryers with automatic loading systems via a conveyor belt; following on to a capping machine to complete the packaging process. There is also an option for both non-lyophilized products and products requiring terminal sterilization to be manufactured, providing improved versatility. The aseptic area integrates some auxiliary installations intended to complete the manufacturing process: raw material dispensing, compounding, washing and preparation for sterilization process.
The plant design, for which the latest technological solutions existing in the markets have been taken into account, responds to a demanding implementation of oRABS isolation technology whereby the product is exposed in order to minimize the contamination risk.
Complete solution
The scope of the project awarded to Telstar incorporates the conceptual, basic and detailed engineering, the construction of a new manufacturing area and the provision of process equipment for pharmaceutical production, almost entirely designed and manufactured by Telstar. The project covers the cleanroom architecture provision, HVAC system fitted with integrated decontamination technology, critical utilities (highly purified water, water for injection using distillation method, pure steam and nitrogen gas), non-critical utilities (compressed air, softened water, chilled & hot water, industrial steam), electricity and the complete automation system, which integrates HVAC control process, decontamination and particles monitoring system, amongst others.
In connection with the pharmaceutical process production equipment, the new plant will be fitted with two liquid nitrogen freeze-dryers with vial automatic loading & unloading systems performing under laminar flow oRABS, and two autoclaves used for material sterilization, and product terminal sterilization, when required. In addition ten items of containment equipment has also been purchased including weighing booths, pass-through boxes integrating biological disinfection system and laminar air flow cabinets. Finally, Telstar will also perform and manage the validation service including risk analysis, design qualification (DQ), commissioning, installation qualification (IQ), operation qualification (OP) and performance qualification (PQ), in addition to support services for quality assurance.
Telstar, a specialist in the development of aseptic manufacturing installations, undertakes both the design and the complete execution of the project from its conception to the final validation, as well as the supply of equipment designed and manufactured using in-house technology. The allocation of the turnkey construction project was agreed in July 2017, after completing the engineering, design and equipment definition phases in May 2017. The complete installation is planned to be finished in the first quarter of 2019.
Energy efficiency
Taking into consideration aspects related to GMP compliance and the latest trends in the industry field, the design of the new pharmaceutical plant has also integrated energy efficiency systems, generating power and production cost savings. The review of the European Pharmacopoeia monograph 169 promoted by the European Medicines Agency (EMA) on water for injection (WFI) , allowing water to be obtained by non-distillation methods from the 1st of April 2017 on, has led Telstar to implement a generation system for WFI using reverse osmosis and ultrafiltration in the new plant for Baccinex. The installation has also got a small production of WFI by distillation.
Baccinex
Headquartered in Courroux (Switzerland), Baccinex is a full-service pharmaceutical contract manufacturing organization (CMO) specialized in filling and finishing of sterile lyophilized or liquid dosage forms. The company cooperates with major international companies offering complete services from pharmaceutical development and manufacturing of clinical trial batches to commercial manufacturing, packaging, analytical services and logistics.

Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance