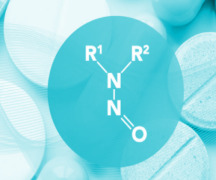
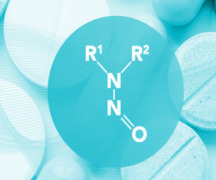
Batch Testing & EU Batch Release / Certification
Product Description
QpLab - Pharma Services, Lda

-
PT
-
2019On CPHI since
-
2Certificates
-
1 - 24Employees
Company types
Categories
Specifications
QpLab - Pharma Services, Lda

-
PT
-
2019On CPHI since
-
2Certificates
-
1 - 24Employees
Company types
More Products from QpLab - Pharma Services, Lda (2)
-
Product Nitrosamines - Quantitive Quality Control
LC-MS/MS methods are nowadays commonly used for nitrosamines analysis in all types of materials, since it allows analyte-specific detection based on both retention time and structurally specific fragmentation information in conjunction with high sensitivity.
After the establishment of the ni... -
Product Nitrosamines - Confirmatory Tests
The screening test consists in the assessment of which nitrosamines are present in the drug product below an acceptable limit, defined according to the recommendations of EMA.
These limits are calculated based on the acceptable intake (AI) of each nitrosamine. QPLAB has a LC-MS/MS method tha...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance