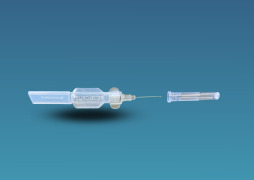
Blow-Fill-Seal
Product Description
SAS LABORATOIRE UNITHER

-
FR
-
2015On CPHI since
-
4Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
SAS LABORATOIRE UNITHER

-
FR
-
2015On CPHI since
-
4Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from SAS LABORATOIRE UNITHER (6)
-
Product Euroject®
Recent innovations have opened up the possibility of using BFS technology to design aseptic packaging systems combining the efficiency of high-throughput production with the highest guarantees of sterility. The result is a new type of pre-filled, single-dose disposable device that can be produced... -
Product Multidose
Preservative-free Multidose (PFMD) & Multidose with Preservative (MDWP)To meet the demands of our customers and the market, we have equipped ourselves with a multi-dose aseptic filling capacity. Unither offers development services and subcontracting of ophthalmic formulas for both PFMD and MDWP te... -
Product Liquid Stick-Pack
Unither is a referent for liquid stick-pack technology thanks to its multidisciplinary and international team.
Liquid stick-packs deliver just the right dose for precise dosage. Packaging protects contents from moisture, oxygen and light.
Thanks to its small size and compactn... -
Product Spray
Unither Liquid Manufacturing (ULM) is a production site of Unither Group. ULM is dedicated to the development and manufacture of health products in semi solid and liquid forms, such as nasal sprays. Nasal spray humidifies, cleans, decongests and purifies the nasal cavities to improve breathing. It is ... -
Product Suppositories & Pessaries
Suppositories and pessaries are an ideal method of administration for a drug when the oral route is not possible due to nausea or vomiting. This is one of the best ways to avoid the first-path effect, which lowers the efficacy of a drug product. Unither has many years of experience in the manufacture of su... -
Product Tablets & Capsules
Unither Pharmaceuticals is able to provide a range of flexible services in the production of tablets and capsules. We can work with you from the earliest stages of R&D to create a new dry form, or we can be your partner in reformulating or packaging an existing product.
Advantages :
qq...
SAS LABORATOIRE UNITHER resources (5)
-
News $106 Million to Modernize Rochester Facility
Unither Pharmaceuticals CEO Eric Goupil said, “We are extremely pleased to benefit from the support of Empire State Development, Monroe County, RG & E, and Greater Rochester Enterprise in extending our facility while reducing our carbon footprint. Unither’s purpose is to provide innovative, competitive, and easy to use solutions to simplify the life of patients around the world while reducing our Carbon footprint and our impact on the environment and taking care of our employees to offer them job opportunities and careers possibilities. This expansion will enable our Rochester plant to increase its capacity on preservative-free sterile products and will allow us to support our customers in their growth. Thanks to the dynamism of all local stakeholders, we are confident to reach a leadership position in the US.” -
News Manufacturing of registration batches of PXT3003 in single unit doses
Pharnext SCA (FR001400JXB0 – ALPHA) (the “Company”), an advanced late-clinical stage biopharmaceutical company developing novel therapeutics for neurodegenerative diseases with high unmet medical need, announces the successful manufacturing of the three registration batches of PXT3003 oral solution sachets in the United States (U.S.), utilizing an innovative and state-of-the-art “form-fill-seal liquid sachets technology” and initiated the new drug application (NDA) registration stability at Unither Manufacturing LLC in Rochester, NY (USA). -
News Scientific Review – Age-related Macular Degeneration (AMD)
This scientific review delves into collaborative research conducted by teams from Unither Développement Bordeaux alongside partners from various institutions including Université Paris Cité, CNRS, INSERM, UTCBS, Unité de Technologies Chimiques et Biologiques pour la Santé, Département de Recherche et Développement (DRDP), Agence Générale des Equipements et Produits de Santé (AGEPS), Assistance Publique Hôpitaux de Paris (AP-HP), as well as the Institute of Pharmaceutical Sciences of Western Switzerland (ISPSO) and the School of Pharmaceutical Sciences at the University of Geneva. -
News BIOPHTA raises 6.5 million Euros Seed Funding to transition its New Standard of Care for Eye Diseases
BIOPHTA raises 6.5 million Euros Seed Funding to transition its New Standard of Care for Eye Diseases to the Clinical stage
The seed round was co-led by UI Investissement (via the Pertinence Invest 2 Fund, advised by Mérieux Equity Partners), Elaia and GO Capital, with participation from one major ophthalmology player, Unither Pharmaceuticals and HTL Biotechnology, the global leader in production and development of pharma grade biopolymers.Proceeds to fund first-in-human trial of breakthrough topical Ophthalmic Inserts in the treatment of Glaucoma and to advance a second program in Macular Edema, thus targeting the two leading causes of blindness.BIOPHTA’s unique technology is poised to disrupt the treatment of both the front- and back-of-the-eye. The company is developing the first continuous topical retina treatment to free patients from the need for intraocular injections.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance