Building resiliency into single-use systems
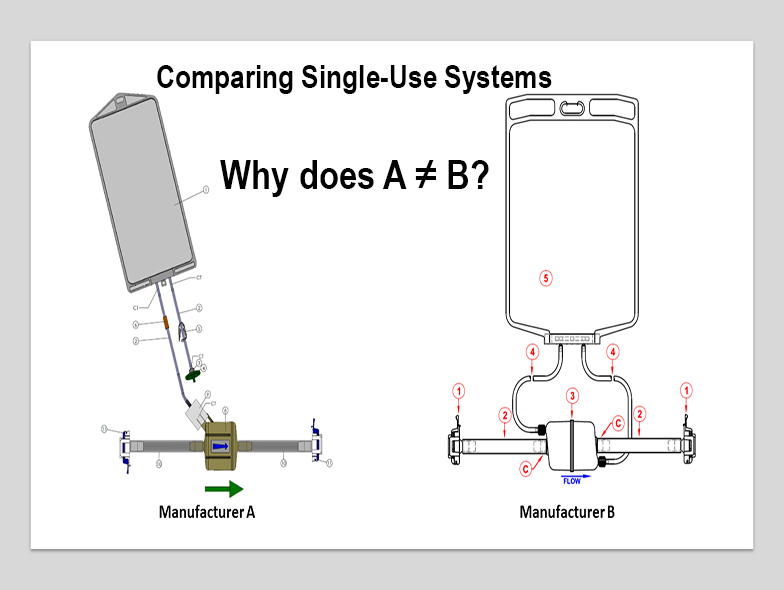
Jo Anne Jacobs, Director of Technical Services at INCOG BioPharma Services, discusses building a functional equivalence model for single-use manufacturing equipment and assemblies for use in drug product manufacturing.
Jo Anne Jacobs, Director of Technical Services at INCOG BioPharma Services
 I have been in the CDMO industry nearly 30 years, meaning I have seen a lot of change. I have always found innovative new technologies exciting and like to jump right in and try it, both in my professional capacity and my personal life. 'There’s a new iPhone? Got to have it!' I am naturally drawn to figuring out the how and why of equipment, processes, etc. and typically have an inclination to improve the status quo.
I have been in the CDMO industry nearly 30 years, meaning I have seen a lot of change. I have always found innovative new technologies exciting and like to jump right in and try it, both in my professional capacity and my personal life. 'There’s a new iPhone? Got to have it!' I am naturally drawn to figuring out the how and why of equipment, processes, etc. and typically have an inclination to improve the status quo.
However, in an industry that is known to be bold, forward thinking, innovative – or is it? 'Change' is a scary word. Everyone is hesitant to be first.
The integration of single-use (disposable) processing equipment has become well accepted in the last 10-15 years. The growth of biological therapies helped spearhead the adoption of disposable bag-based bioreactors, filtration, cartridges/capsules, etc. for use in drug substance manufacturing. The main drivers being: lower cost for initial set-up (compared to stainless steel tanks), faster change over, less cleaning validation, and smaller footprint to accommodate more individualized/small batch therapies.
All of these drivers are key to moving a product forward faster while still maintaining high quality standards. Drug product manufacturing was a little slower to adopt these advances. We took baby steps – disposable capsule filters, peristaltic pump filtration and filling assemblies. The mixing tanks and holding vessels were a little bit harder sell – but we got there. One of the main upsides of single-use mixing systems was that the lead times for procurement were much quicker than for stainless steel tanks – but then came COVID-19. The lead times for single-use equipment increased substantially. So much so that a move back to stainless steel was looking more and more attractive.
The concept of 'functional equivalence' has been accepted in clinical practice with respect to drug products for many years. This is different from substituting a 'generic' version of a brand-name product. In this case, a completely different active substance is substituted for the product ordered by the physician. This didn’t happen overnight, as a controlled and well-defined process for this had to be developed and proven to be safe. Being a pharmacist, I have always had a difficult time understanding why this concept couldn’t be applied in pharmaceutical manufacturing.
I want to plant the seeds for developing a functional equivalence model for single-use manufacturing equipment and assemblies for use in drug product manufacturing.
One of the main faults with implementing single-use systems is a lack of knowledge. This is not to imply that there is a dearth of actual information, but that there has been little coordination between the various end-users and the vendors developing the single-use items.
For example, let’s look at pickup trucks. A Ford, Chevrolet or Ram are different in many ways, but in general can each perform the same function. The potential buyer looks at what each has to offer (from the basics to the bells and whistles) and determines which will best suit their needs. But, if you decide that one of the primary specifications for your truck is that it be made by the Ford Motor Company, you greatly limit your choices.
In the pharmaceutical industry we have done essentially that for years. In our regulatory submissions, we often identify equipment and materials down to the specific manufacturer and often the manufacturing site. That’s how 'it’s always been done'.
We look at the vendors/suppliers as just that, someone who provides us with commodities we need to manufacture life-saving therapies. The vendors are working to create and design equipment and materials that they can convince the pharmaceutical industry it 'needs'. In many cases, the final decision is based less on scientific knowledge and more on previous familiarity with the vendor/supplier of the item. We all know that you are not going to convince a Ford F-150 owner that a Chevy Silverado is equivalent, but functionally they are.
The debate over how much regulation/standardization is too much is never-ending. But the time for developing an agreement on defining functional equivalence for single use materials is now. To do that the pharmaceutical industry, the suppliers to the pharmaceutical industry, and the various regulatory bodies will need to work together. Each brings substantial subject matter expertise to the discussion. All of this knowledge taken together and used appropriately will result in a more robust supply chain and ability for the pharmaceutical industry to avoid the often-self-imposed roadblocks we ran into over the past two years. There is no scientific reason to believe that one manufacturer’s sterilising filter (polypropylene capsule, PVDF 0.22 micron membrane) is not equivalent to another manufacturer’s item of the same description. So long as the supplier meets the requirements of the end- user’s quality systems, they should be able to be used interchangeably. We just need to develop the system that makes this possible.
In general, the approach to single-use has been to design and qualify a 'system' of components based on the specific application. This results in the generation of data that often cannot be shared across applications because it is proprietary to a specific company/product.
Developing a list of performance attributes and qualifying tests (common user requirements, if you will) would serve several purposes:
- The ability to directly compare items from various vendors
- Decrease the number of requests for information the vendors have to address
- Potential to decrease the number of sub-units/suppliers the vendors must maintain
- Decreased inventory costs
- Ability to use alternate suppliers without extensive requalification, which saves time and money
- Less customization leads to shorter overall project timelines
To make this happen, a number of steps must be taken. Standardization of connections so that the various pieces and parts of the complete system can come from multiple vendors is a critical step (the LEGO® approach). Even if we prove that filters from two different vendors are equivalent, we still have a problem if they both can’t be connected to the mixing and holding bags being used in a manufacturing process.
The first reaction from the vendors may be that this would potentially decrease the competitive market space. In reality, even though revenues from compatibility/validation studies may decrease, the ability to implement single-use faster and more cost effectively will lead to more widespread use. This would benefit both the vendors, end users, and ultimately, patients.
This approach is especially attractive to the CDMO/CMO industry. Technology transfer can be very time-consuming and expensive. In situations such as a pandemic, where the ability to provide necessary life-saving medications across the world is vital, being able to implement manufacturing more quickly in multiple sites is crucial. Instead of qualifying an assembly or group of assemblies (single- use-system, SUT), a process can be built by choosing from a pool of qualified parts.
Movement to the more standardized approach won’t eliminate certain product-specific testing that would be needed. But it would make it easier for the end user to determine what that testing may be. Additionally, the testing would not have to be conducted multiple times in order to qualify an alternative.
Probably the biggest hurdle will be convincing industry and regulators to accept functional requirements for materials used for manufacturing rather than specific items. This won’t be easy, but to avoid or minimize the supply chain issues of the recent past (and not yet completely resolved) we have to think differently. A lot of innovative thinking went into meeting the COVID-19 challenge. Now is not the time to revert to the way things were. Taking those learnings and developing solid plans for the future has to happen. There was unprecedented cooperation and open-mindedness among the pharmaceutical industry, regulatory agencies, and vendors during this time. We need to take this opportunity to really embrace innovation.
If implemented properly, the ability to produce even safer, high quality, life-saving medicines even faster will be realised. And that is why this industry exists.

Related News
-
Sponsored Content Ashwagandha and Herbal Medicines: Pharma’s Next Big Opportunity
Herbal medicines and nutraceuticals have seen a surge in interest since the onset of the COVID-19 pandemic. Driven by patient interest in prioritising personalised and integrative medicines, the herbal ingredients industry is now faced with concerns pe... -
Sponsored Content Discover Our Organic Mineral Salts as APIs
Discover the range of organic mineral salts that serve as Active Pharmaceutical Ingredients available from Dr. Paul Lohmann®. -
Sponsored Content CPHI Podcast Series: Ursatec – celebrating 30 years of pioneering preservative free
In the latest episode of the CPHI Podcast Series, Digital Editor Lucy Chard spoke with Dominik Rocchi of Ursatec. -
Sponsored Content How healthcare trends inform dosage forms
Capsules encompass one of the most popular solid oral dosage forms for pharmaceutical products, with the global empty capsule market predicted to rise to USD $3.7 billion by 2026. The growth in the capsule market can be partly attributed to the many op... -
Sponsored Content 2023 Pharma Trend Outlook: Innovation, Resilience, and Pharma 4.0
Download our 2023 Pharma Trends Outlook report to discover the trends set to shape the pharmaceutical landscape in the new year, with expert opinions and insight from across the pharmaceutical value chain. -
Sponsored Content CPHI Podcast Series: Key Considerations in Selecting the Right CMO Partner
In this month's episode we hear from Jayna Blake, Senior Project Manager for Technical Programs at Baxter BioPharma Solutions, on key considerations for successful CMO selection. -
Sponsored Content Size doesn’t matter: How smaller deals are shaping healthcare M&A
Several mega-deals have made a splash in the pharma and life sciences industries over recent years – from AstraZeneca’s acquisition of Alexion for $39 billion to Gilead Sciences’ $21 billion purchase of Immunomedics. With am... -
Sponsored Content Rise in home-based healthcare shaping pharma packaging and drug delivery
The pharma packaging and drug delivery industry has long been synonymous with rapid innovation. As medicine advances and patient needs change, so too should drug packaging and devices. This has been particularly evident during the pandem...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
.png)