Cancer, rare diseases and revenue eclipse COVID-19: The drug approval drivers in 2020

COVID-19 has been a major focus for drug regulators on both sides of the Atlantic in 2020. But efforts to help industry combat the pandemic did not appear to have had a major impact on review activities judging by the drugs approved so far this year
Despite the major focus on developing vaccines and treatment for COVID-19, 2020 has seen some new trends in drug approvals emerge with growth of the rare diseases market and the increased use of patient subsets in the oncology space being the most interesting.
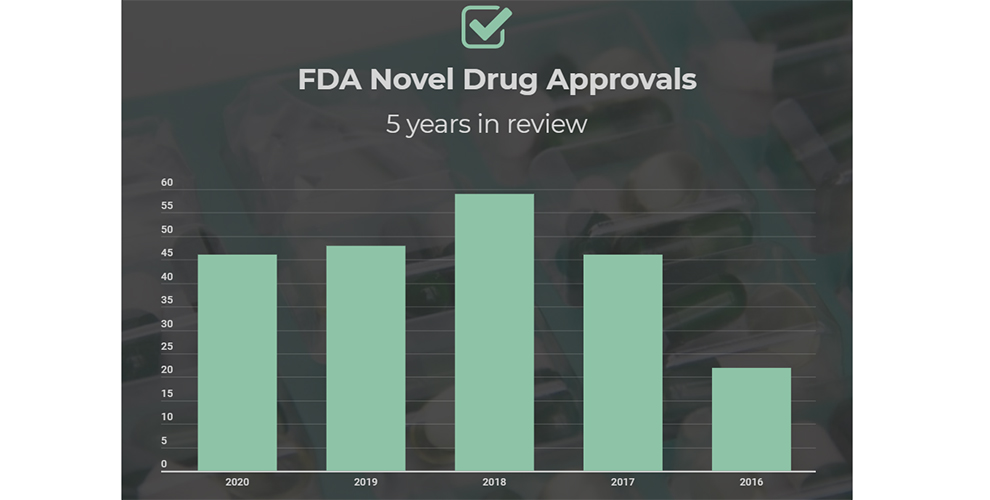
As of mid-November the US Food and Drug Administration (FDA) had approved 46 new molecular entities (NME) in 20201, which is just two fewer than the 48 products2 cleared last year and above the 43 per year average seen since 2015.
The figures are similar in the European Union. The European Medicines Agency’s (EMA) committee for medicinal products for human use (CHMP), the body that recommends whether a drug should be approved, had issued positive opinions on 43 products between January and the middle of last month3.
The European Commission (EC) does not always follow CHMP recommendations, but it usually does. By mid-November 34 of the 53 medicines recommended for approval had been granted marketing authorisation according to publicly available information4. In 20195, 30 new drugs were given the green light in Europe.
In particular, 2020 has been a good year for drug innovators, according to Fiona Barry, Associate Editor at Global Data’s PharmSource.
“Over the January-November 2020 period, 9% more innovative drugs received their first approval from the FDA, EMA, or MHLW [in Japan) than over the same months in 2019," she says. “The top therapy area for approvals was central nervous system drugs, at approximately 20% of approved therapies. Approvals for cardiovascular, infectious disease, and oncology drugs were also high. "
Traditional medicines continued to dominate regulatory decision making, according to Barry: “More than 90% of these approvals were for small molecules. Monoclonal antibodies came a distant second.”
Barry points out that “Gilead/Kite’Tecartus was the year's only cell gene therapy approval, excluding expanded indications for already approved cell/gene therapies.”
Rare diseases
Therapies for rare diseases – defined as those impacting fewer than 200,000 people in the US and no more than one person in 2,000 in the EU – made up a significant proportion of approvals and approval recommendations in 2020.
In the EU notable examples included: Alnylam Pharmaceuticals’ acute hepatic porphyria drug Givlaari6 (givosiran); Novo Nordisk’s type 2 diabetes treatment Rybelsus7 (semaglutide); and Novartis’ spinal muscular atrophy gene therapy Zolgensma8 (onasemnogene abeparvovec). All three products were approved by the FDA last year.
In all, 21 of the 53 products recommended for approval by the CHMP were orphan drugs. Some notable examples expected to receive a final decision this year include Orchard Therapeutics’ Libmeldy9, a cell therapy for a metachromatic leukodystrophy, and Gilead’s Tecartus10, an autologous anti-CD19-transduced CD3+ cells for mantle cell lymphoma.
Rare disease therapies approved In the US included Tecartus, which was cleared in July11, Roche’s neuromyelitis optica spectrum disorder medicine, Enspryng (satralizumab-mwge); and Regeneron’s combination Ebola treatment Inmazeb.
The US FDA also cleared NS Pharma’s Viltepso (viltolarsen) for Duchenne muscular dystrophy; Incyte’s bile-duct cancer pill, Pemazyre (pemigatinib) and Horizon Therapeutics’ thyroid eye disease treatment, Tepezza (teprotumumab-trbw) in 2020.
Revenue generators
The year also saw medicines designed to treat more common diseases win regulatory approval in the US and EU.
Trodelvy, Immunomedics’ treatment for breast cancer was perhaps the highest profile. The US FDA approved it to treat metastatic triple-negative breast cancer in April12.
At the time, Richard Pazdur, director of the FDA’s Oncology Center of Excellence, said Trodelvy “represents a new targeted therapy for patients living with this aggressive malignancy.” Analysts expect the drug to generate revenue of USD 2.13 billion a year by 2026.
According to Reuters,13 Immunomedics – which is due to be acquired by Gilead Sciences – plans to seek approval for Trodelvy in the EU next year.
Daiichi Sankyo and Esperion Therapeutics’ Nexletol – a treatment for cholesterol and cardiovascular disease — is also expected to generate significant revenues. The FDA approved the drug in April14 with the European Commission approving it – as Nilemdo15 – a month later. Analysts predict the drug will generate around USD 2 billion a year in revenues within five years.
Other products to win approval in 2020 include Bristol Myers Squibb’s multiple sclerosis treatment, Zeposia – which was cleared by the FDA in March16 and the EC in May17 - and Aimmune’s peanut allergy treatment, Palforzia, which was approved by the FDA in January and granted a CHMP recommendation in October18.
Oncology
As with in previous years, a significant proportion of drugs approved in 2020 are designed to treat cancer. Jazz Pharmaceutical’s Zepzelca (lurbinectedin) – cleared in the US in June19 – is one of the most eye-catching examples.
The Jazz drug is the first new option in 20 years for small-cell lung cancer patients for whom chemotherapy has failed. Analysts predict it will generate annual revenue of around USD 200 million by 2024.
Eli Lilly’s Retevmo, which was cleared by the FDA for non-small-cell lung cancer and thyroid cancer in May20 – is another noteworthy entrant in the oncology space.
The drug is intended for patients whose tumours have an alteration – a mutation or fusion – in the RET gene. Retevmo is the first therapy approved specifically for cancer patients with the RET gene alterations.
Trodelvy – discussed above – was not the only breast cancer drug approved in the US this year. The FDA also cleared 21 Seattle Genetics’ Tukysa (tucatinib) in combination with chemotherapy for the treatment of advanced forms of HER2-positive tumours.
While Tukysa is not expected to generate revenues in excess of USD 2 billion like Trodelvy, the drug is likely to be a major revenue generator for Seattle Genetics. According to the Fool,22 it is likely to bring in USD 1.2 billion a year by 2030.
Other new oncology drugs include Incyte’s bile duct cancer treatment, Pemazyre (pemigatinib)23 and its metastatic non-small-cell lung cancer product, Tabrecta; as well as Deciphera Pharmaceuticals’ Qinlock (ripretinib) and Blueprint Medicine’s Ayvakit (avarpritinib), which each treat gastrointestinal stromal tumours.
It is interesting to note that many of the oncology products approved this year target specific subsets of patients based on genetic mutations. Qinlock and Ayvakit both inhibit cancers caused by mutant KIT and PDGFRα kinases, however the latter product only targets patients with PDGFRα exon 18 mutation.
Neurodegenerative disease
Some notable therapies for neurodegenerative diseases with few treatment options were also among the new products this year.
The FDA approved24 Neurocrine Biosciences’ drug Ongentys for the treatment of Parkinson’s disease sufferers who are experiencing off periods. The decision – which permits use of once-daily oral Ongentys as an add-on treatment to levodopa/carbidopa – follows four years after the drug was approved in the EU25.
The US regulator also cleared26 Genentech’s Evrysdi (risdiplam) as a treatment for spinal muscular atrophy (SMA).
The Genentech product – which is set to cost USD 340,000 a year – is an oral treatment for SMA. According to the FDA, it is an alternative to both Biogen’s Spinraza27 which costs USD 750,000 a year and Novartis’ USD 1.9 million one-time only treatment gene therapy Zolgensma28.
COVID-19
At the time of writing29, 48 candidate vaccines against SARS-CoV-2, the virus that causes COVID-19, are in clinical development with a further 164 in animal studies. Positive data from some of the most advanced candidates – specifically those being developed by Pfizer30 and Moderna31 – could result in approvals in the near future.
The COVID-19 therapeutics field is much smaller. To date only one treatment – Gilead’s Veklury (remdesivir) has been approved. Several products such as Regeneron’s32 casirivimab and imdevimab antibody cocktail and Eli Lilly’s bamlanivimab33 have been granted emergency use authorisation (EUA)
The Lupus drug Hydroxychloroquine was also granted an EUA; however, authorisation was later withdrawn by the FDA, which cited a lack of evidence of efficacy and concerns about potential side effects34.
End of year
While 2020 is unlikely to match the record 59 new approvals seen two years ago35, the year may yet surpass the 50 mark.
Approval decisions for BioCryst Pharmaceuticals’ hereditary angioedema drug Berotralstat; Ainylam Pharmaceuticals’ primary hyperoxaleria type 1 treatment Lumasiran; and Myovant Sciences prostate cancer therapy, Relugloix, are all expected before the end of the year.
Likewise, Fibrogen and AstraZeneca say the FDA will decide whether or not to clear the chronic kidney disease candidate Roxadustat just before Christmas and Pfizer and Eli Lilly are due to hear about their candidate osteoarthritis monoclonal antibody (mAb) Tanezumab before the new year.
Next year anti-infectives surge
The industry’s focus on COVID-19 likely to have more of an impact on the approval landscape in 2021 according to Barry, who also predicts there will be a knock-on impact for the contract services sector.
“COVID-19 vaccine developers are signing contract service agreements for pipeline vaccines at an unprecedented rate for a novel indication. Consequently, infectious disease drugs have already overtaken perpetual leader oncology as the year’s top therapy area for contract manufacturing service agreements.”
Barry adds that, “Even the largest companies require extra resources to supply billions of doses with AstraZeneca and Inovio having the largest number of Contract Service Agreements to date.
“Pharma companies such as Pfizer are also pushing internally manufactured drugs out to CMOs to free up space for COVID-19 vaccine manufacture.”
Gareth Macdonald
References:
- https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020
- https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019
- https://www.ema.europa.eu/en/committees/chmp/chmp-agendas-minutes-highlights
- https://ec.europa.eu/health/documents/community-register/html/reg_last.htm
- https://www.ema.europa.eu/en/documents/report/human-medicines-highlights-2019_en.pdf
- https://www.businesswire.com/news/home/20200303005205/en/Alnylam-Announces-Approval-of-GIVLAARI%C2%AE-givosiran-in-the-European-Union-for-the-Treatment-of-Acute-Hepatic-Porphyria-AHP-in-Adults-and-Adolescents
- https://www.ema.europa.eu/en/medicines/human/EPAR/rybelsus
- https://www.novartis.com/news/media-releases/avexis-receives-ec-approval-and-activates-%22day-one%22-access-program-zolgensma-only-gene-therapy-spinal-muscular-atrophy-sma
- https://www.ema.europa.eu/en/medicines/human/summaries-opinion/libmeldy
- https://www.ema.europa.eu/en/medicines/human/summaries-opinion/tecartus
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-cell-based-gene-therapy-adult-patients-relapsed-or-refractory-mcl#:~:text=Today%2C%20the%20U.S.%20Food%20and,following%20other%20kinds%20of%20treatment.
- https://www.fda.gov/news-events/press-announcements/fda-approves-new-therapy-triple-negative-breast-cancer-has-spread-not-responded-other-treatments#:~:text=Today%2C%20the%20U.S.%20Food%20and,prior%20therapies%20before%20taking%20Trodelvy.
- https://fr.reuters.com/article/idUSKBN2640V8
- https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/211616Orig1s000Approv.pdf
- https://www.globenewswire.com/news-release/2020/08/26/2084098/0/en/Esperion-Announces-Two-Data-Presentations-of-NEXLETOL-bempedoic-acid-Tablet-at-the-ESC-Congress-2020.html#:~:text=NEXLETOL%20was%20approved%20by%20the,NILEMDO%E2%84%A2%20(bempedoic%20acid).
- https://news.bms.com/news/details/2020/US-Food-and-Drug-Administration-Approves-Bristol-Myers-Squibbs-ZEPOSIA-ozanimod-a-New-Oral-Treatment-for-Relapsing-Forms-of-Multiple-Sclerosis/default.aspx
- https://www.ema.europa.eu/en/medicines/human/EPAR/zeposia
- https://ir.aimmune.com/news-releases/news-release-details/aimmune-receives-positive-chmp-opinion-palforziar-treatment
- https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-lurbinectedin-metastatic-small-cell-lung-cancer
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-patients-lung-and-thyroid-cancers-certain-genetic-mutation-or-fusion
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-new-drug-under-international-collaboration-treatment-option-patients-her2
- https://www.fool.com/investing/2020/04/23/is-it-too-late-to-buy-shares-of-seattle-genetics.aspx
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-treatment-patients-cholangiocarcinoma-cancer-bile-ducts
- https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/212489Orig1s000Approv.pdf
- https://www.ema.europa.eu/en/medicines/human/EPAR/ongentys
- https://www.fda.gov/news-events/press-announcements/fda-approves-oral-treatment-spinal-muscular-atrophy
- https://xconomy.com/boston/2016/12/28/biogen-sets-750000-initial-price-for-first-ever-spinal-atrophy-drug/
- https://www.forbes.com/sites/joshuacohen/2019/06/05/at-over-2-million-zolgensma-is-the-worlds-most-expensive-therapy-yet-relatively-cost-effective/
- https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- https://www.marketwatch.com/story/pfizer-biontech-vaccine-could-be-approved-within-days-with-u-k-set-to-be-first-to-start-covid-19-shots-11606741593
- https://www.sciencemag.org/news/2020/11/absolutely-remarkable-no-one-who-got-modernas-vaccine-trial-developed-severe-covid-19
- https://www.prnewswire.com/news-releases/regenerons-regen-cov2-is-first-antibody-cocktail-for-covid-19-to-receive-fda-emergency-use-authorization-301178464.html
- https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
- https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
- https://cen.acs.org/pharmaceuticals/drug-development/new-drugs-2018/97/i3
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance