CDMO service
Product Description
Samyang Biopharmaceuticals Corp

-
KR
-
2016On CPHI since
Categories
Samyang Biopharmaceuticals Corp

-
KR
-
2016On CPHI since
More Products from Samyang Biopharmaceuticals Corp (2)
-
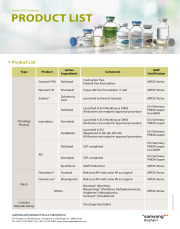
Product APIs
[Cytotoxic APIs]
Paclitaxel Produced by fermentation
Paclitaxel Produced by a semi-synthetic process
Docetaxel, anhydrous
Pemetrexed
Bortezomib
Cabazitaxel
Package: Up to 500g per request
*If you need further information on our APIs,... -
Product FDFs
Oncology products:
Genexol PM (Paclitaxel)
Nanoxel M (Docetaxel)
Zolenic (Zoledronic acid)
Injectables (Paclitaxel, Docetaxel, Oxaliplatin)
Patch Products:
Fentaderm (Fentanyl)
Demencure (Rivastigmine)
Nicostop (Nicotine)
Rheumastop (Diclofenac Diethylammonium)
Anigderm (Nitro...
Samyang Biopharmaceuticals Corp resources (2)
-
News Samyang Biopharm USA in-licenses Talix Therapeutics’ first-in-class compound
Samyang Biopharm USA has licensed in Belgium’s Talix Therapeutics’ first-in-class immuno-oncology compound for global rights. -
Brochure Pharmaceuticals Product List Brochure
If you would like to know more about our products, please click our brochure!
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance