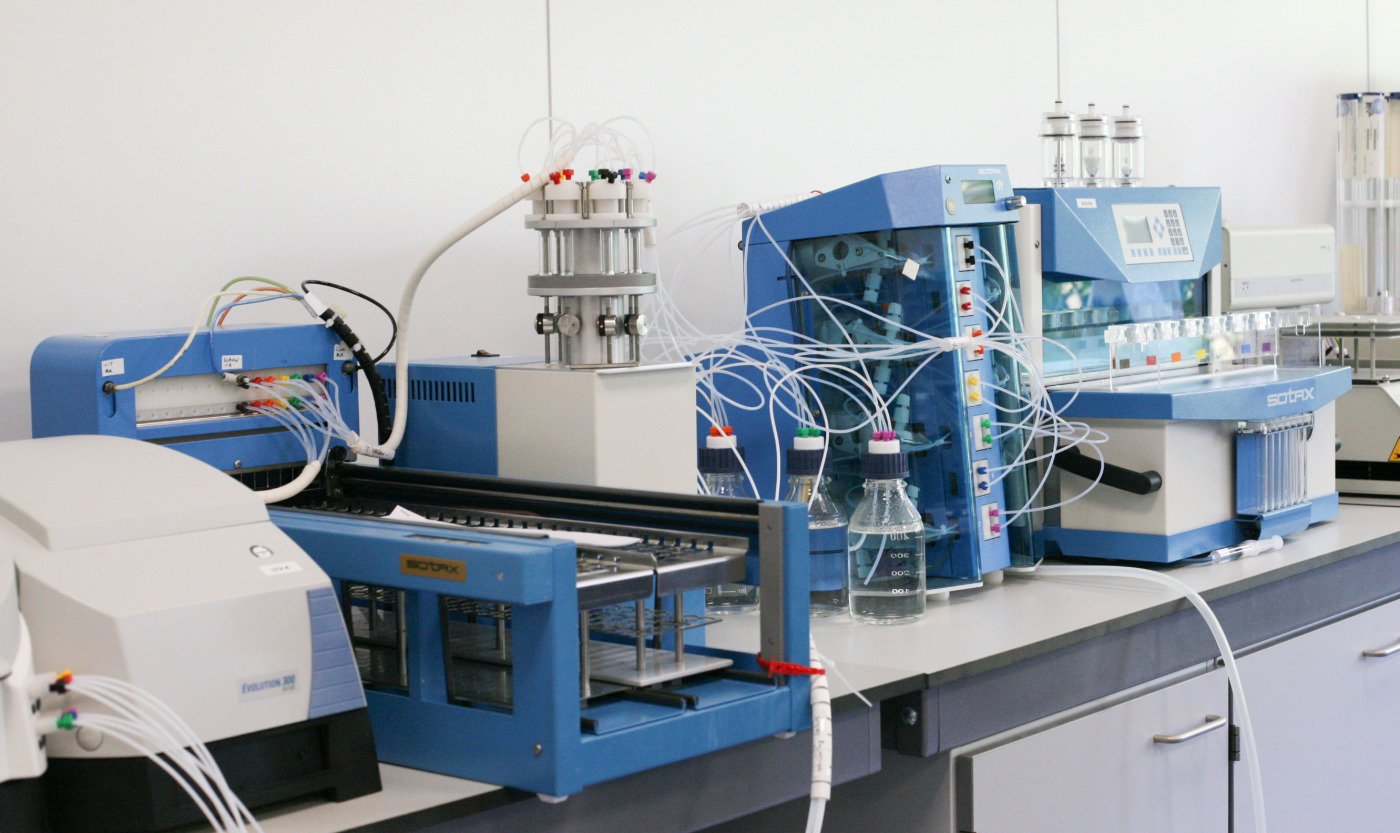
Dissolution Testing - R&D services
Product Description
Avivia BV

-
NL
-
2017On CPHI since
-
1 - 24Employees
Company types
Primary activities
Categories
Specifications
Avivia BV

-
NL
-
2017On CPHI since
-
1 - 24Employees
Company types
Primary activities
More Products from Avivia BV (4)
-
Product Excipia Experts in excipient analysis, characterization and deformulation / reverse engineering
Excipients can have an important impact on the manufacturability and pharmaceutical performance of a formulation. It’s therefore important to identify and control functionality related characteristics (FRCs) of excipient materials in order to achieve safe, robust and stable products. Expe... -
Product Pharmaceutical R&D Services
Having decades of hands-on experience on a wide range of (complex) technologies and a many different types of products, our Pharmaceutical experts are able to fully execute and/or support the development of any finished dosage form, formula or process, as well as the transfer and scale-up of a deve... -
Product Analytical research & development
Our analytical experts know what it takes to bring a pharmaceutical product to the market, from discovery, feasibility to GMP. Having worked with more than 100 unique molecules and even more formulations, we may state that we have faced the most difficult CMC challenges that are involved.
... -
Product Excipient Quality Testing and Selection Services
Excipient testing, composition and variabilityExcipients are either natural / naturally derived or synthetic / semi-synthetic. In all cases the exipients are obtained through chemical processing of a raw material source that usually has an animal, vegetable or mineral origin.
...
Avivia BV resources (1)
-
Brochure Avivia Services
Brochure Avivia independent pharmaceutical development services.
Established in 2005, Avivia is a specialized pharmaceutical development company engaged in CRO service activities, drug repositioning, repurposing and reformulation. Over the past fifteen years, the company has built a solid reputation recognized for its R&D flexibility and ‘out-of-the-box’ approach towards complex and challenging analytical and pharmaceutical development projects. Today, we are the preferred partner for many big pharma, specialty pharma, generic drug companies, academic centra, and smaller drug development firms.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance