EMA request for Nitrosamine Risk Assessment
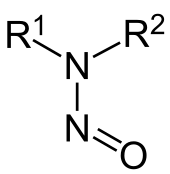
The European Medicines Agency (EMA) requests Marketing Authorization Holders to perform a Risk Assessment for the presence of Nitrosamines in all Chemically Synthesized API’s.
The European Medicines Agency (EMA) requests Marketing Authorization Holders to perform a Risk Assessment for the presence of Nitrosamines in all Chemically Synthesized API’s.
Nitrosamines are classified as probable human carcinogens. Due to findings in multiple medicines since July 2018, EMA recently published an urgent request (see press release of 26/09/2019). As a precautionary measure, all Marketing Authorization Holders are asked to review their manufacturing processes and evaluate the presence of nitrosamines in their medicinal product.
This evaluation should be performed for every concerned medicine within 6 months.
Ardena can help you performing the nitrosamine risk assessment for any synthesized API to stay compliant and to keep ensuring the safety of the patients.
We would be pleased to make an appointment to present our solutions on this matter.

Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance