GMP Consultation

Product Description
Beijing KingRaBroad (KRB) Consulting Inc

-
CN
-
2022On CPHI since
-
25 - 49Employees
Company types
Categories
Beijing KingRaBroad (KRB) Consulting Inc

-
CN
-
2022On CPHI since
-
25 - 49Employees
Company types
More Products from Beijing KingRaBroad (KRB) Consulting Inc (2)
-
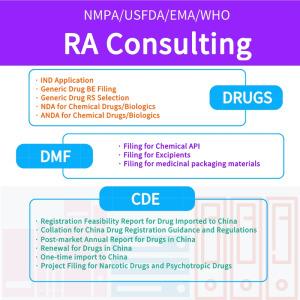
Product Regulatory affairs services
I. SERVICES 1. Submission of ANDA, NDA and IND applications with NMPA; (including the whole service from GAP analysis, compiling, submission, sample testing, CDE review procedure tracking and supplemental info preparation); 2. GAP analysis and application strategies setup before the submission to NMPA; 3. ... -
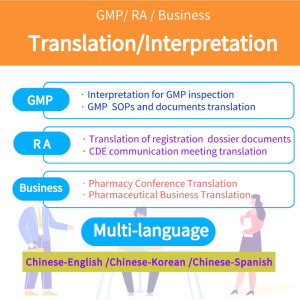
Product Translation services
We have professional translators that can provide: - English & Chinese interpretation services during international GMP audits; - English & Chinese interpretation services during communication with NMPA and other authorities; - English & Chinese translation of audit reports / CAPA reports; - En...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance