Implications of calculating the PDE as the exposure limit for the analysis of risks in shared installations
Socosur

-
FR
-
2015On CPHI since
-
1Certificates
-
1 - 24Employees
Other Content from Socosur (5)
-
News Tox By Design Chosen for BioJapan 2023
Tox By Design has been selected by the EU-Japan Centre for Industrial Cooperation as one of the top 10 European pharmaceutical companies to participate in Asia's Premier Partnering event in Yokohama, Japan!
Asia's Premier Hybrid Partnering event in Yokohama will consist of three exhibitions:
BioJapan - the world's oldest biotechnology exhibitionRegenerative Medicine JAPAN - an exhibit aiming to accelerate and industrialize R&D in the field of regenerative medicine
Health TECH JAPAN - a fusion exhibit of digital technology and life science.
We are so excited to be attending BioJapan 2023 and look forward to further expanding our toxicological expertise within Asia. Feel free to contact us to set up a meeting at our booth from October 11th-13th! -
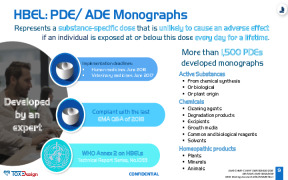
Whitepaper Permitted Daily Exposure (PDE) Monograph
Tox By Design offers Permitted Daily Exposure (PDE) monographs for active pharmaceutical substances, cosmetics, and medical devices! More than 1,500 PDE monographs have already been developed internally by the Tox By Design expert team, all signed by a European Registered Toxicologist (ERT). However, we can develop any PDE monograph for you on demand, including new processes for plant extracts and homeopathic products. -
Whitepaper Occupational Exposure Limit (OEL) Monograph
Tox By Design offers Occupational Exposure Limit (OEL) monographs for active pharmaceutical substances, cosmetics, and medical devices! More than 1,000 OEL monographs have already been developed internally by the Tox By Design expert team, all signed by a European Registered Toxicologist (ERT). However, we can develop any OEL monograph for you on demand, including new processes for plant extracts and homeopathic products. -
Whitepaper Toxicological Expertise for Biotech
Tox By Design offers a range of toxicological expertise to ensure the safety and efficacy of your pharmaceutical and biotech products. Our expert toxicological team offers assistance to biotech companies during the industrial implementation of New Chemical Entities and New Biological Entities following ICH guidelines through toxicological risk assessment and evaluation.
We can guide you through every step of the EU’s mandatory ICH impurity report and provide assistance if you have an impurity that goes out of specification.
Tox By Design can also assist you by:- Calculating a temporary HBEL using in-silico (QSAR) results
- Updating your HBEL as toxicology study results become available
- During New Chemical Entity (NCE) or New Biological Entity (NBE) development, we can also develop & update OELs, PDEs and SDSs packaging
-
Whitepaper Toxicological Expertise for Biotech
Tox By Design offers a range of toxicological expertise to ensure the safety and efficacy of your pharmaceutical and biotech products. Our expert toxicological team offers assistance to biotech companies during the industrial implementation of New Chemical Entities and New Biological Entities following ICH guidelines through toxicological risk assessment and evaluation.
We can guide you through every step of the EU’s mandatory ICH impurity report and provide assistance if you have an impurity that goes out of specification.
Tox By Design can also assist you by:- Calculating a temporary HBEL using in-silico (QSAR) results
- Updating your HBEL as toxicology study results become available
- During New Chemical Entity (NCE) or New Biological Entity (NBE) development, we can also develop & update OELs, PDEs and SDSs packaging
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance