Infusion Bottles, Bags and Hanger Labeling
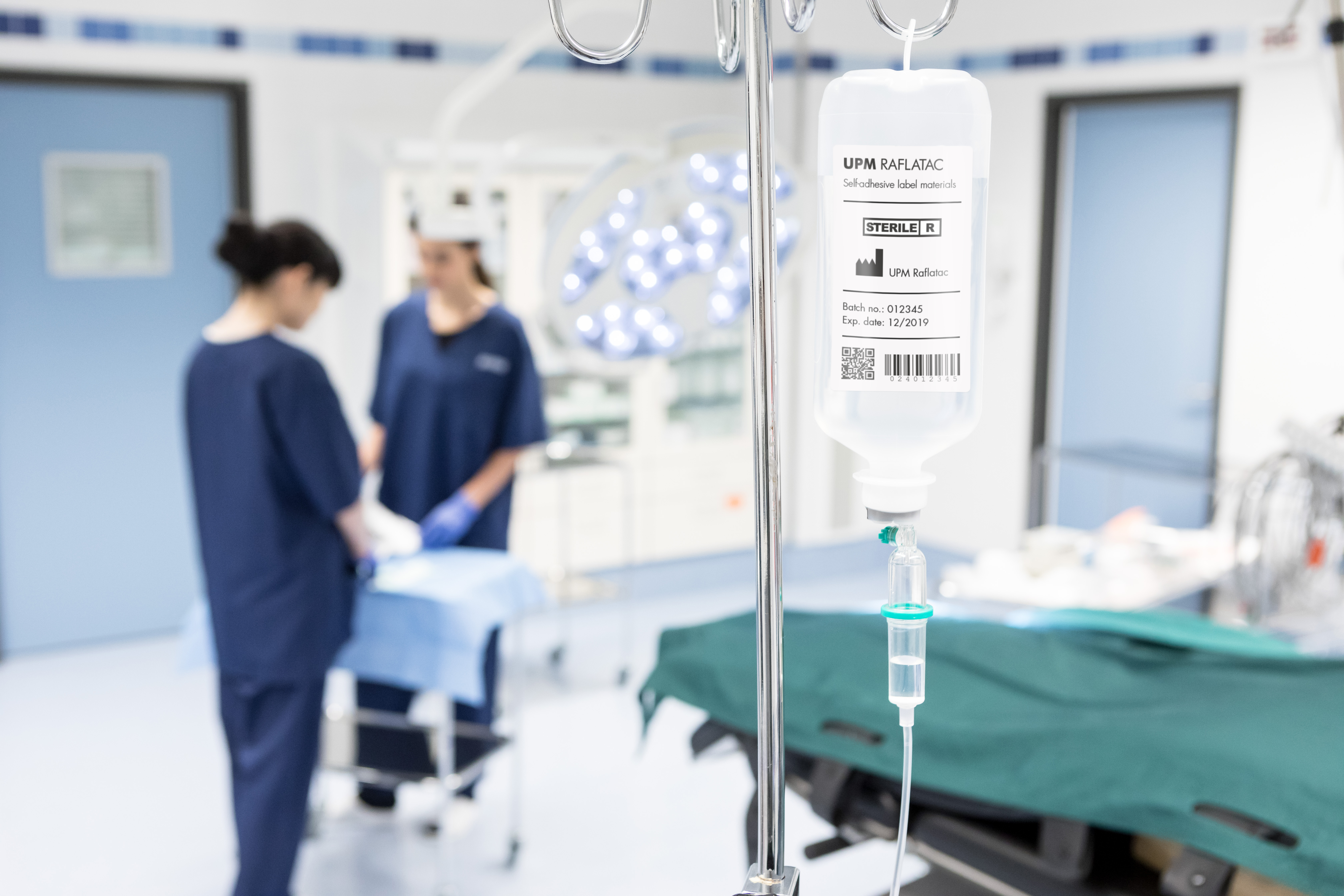
Product Description
UPM Raflatac

-
US
-
2018On CPHI since
-
1Certificates
-
1000 - 4999Employees
Company types
Categories
UPM Raflatac

-
US
-
2018On CPHI since
-
1Certificates
-
1000 - 4999Employees
Company types
More Products from UPM Raflatac (2)
-
Product Cold and Cryogenic Labeling
UPM Raflatac label materials based on the RP Cryo adhesive withstand cryopreservation conditions down to -321 °F (-196 °C), in both vapour phase and liquid nitrogen. The adhesive is extremely resistant to thermal shock, and provides a strong and enduring bond between the label face and PP plastic container... -
Product Hospital and Medical Device Sterilization Package Labeling
Self-adhesive labels are relied on to apply with complete ease and efficiency in countless scenarios. Why should sterilization wraps be any different? UPM Raflatac’s RP3H adhesive puts the ‘self-adhesive’ back into the label. It retains excellent adhesion in the steam, pressure, wetness and heat of the aut...
UPM Raflatac resources (1)
-
Brochure Labeling Solutions Brochure
This brochure gives an overview of common pharmaceutical and healthcare applications and the UPM Raflatac label solutions that meet these various needs. Every pharmaceutical packaging and labeling solution must comply with strict industry regulations. UPM Raflatac pharmaceutical label materials meet or exceed the following regulatory and safety requirements:EMA (European Medicines Agency)/CVMP/205/04 Guideline on plastic immediate packaging materials 19 May 2005 FDA (Food and Drug Administration, USA) Guidance on Container Closure Systems for Packaging Human Drugs and Biologics (1999) Compliance with 1907/2006/EC (REACH) European food contact regulations; Food Framework Regulation 1935/2004,plastics regulated by EU 10/2011 FDA food contact compliance: FDA 21 CFR 175.105 and 175.125 for adhesives; FDA 21 CFR 176.170 and 176.180 for papersFalsified Medicines Directive (2011/62/EU
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

