Innovation in Drug Delivery - Part Two: Therapy area trends for injectables

By Yifei Chen and Yasemin Bettina Karanis, IQVIA
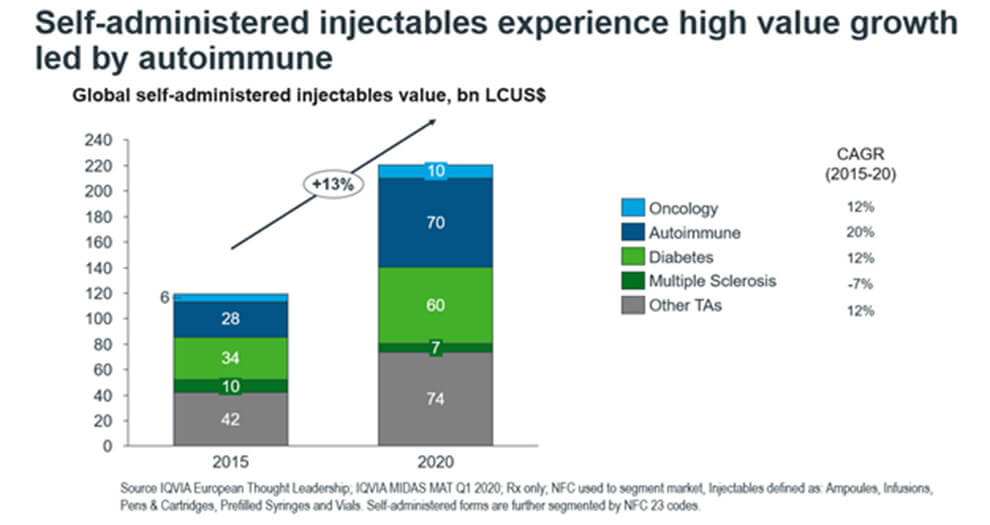
In our previous article, we identified that the leading therapy areas for self-administered injectables are autoimmune, antidiabetics, oncology, and multiple sclerosis (Figure 1).
In this article we’ll explore the individual market dynamics a little further. Let us begin with the highest-value self-administered segments – autoimmune and diabetes. Diabetes is an area where we see some of the most innovative injectable delivery systems. This is due to the insulin market having matured over the many past decades. The treatment area, chronic in nature, has long demanded patients to have the freedom to self-administer. Hence, the injectable diabetes market is nearly completely saturated with self-administered products, and further innovation aims to be more patient centric by looking beyond formulation, into digital and connective devices.
Figure 1. The split in self-administered injectables market into top therapy areas
The development of the injectable autoimmune market, however, is more novel and has a higher growth rate in the trend to create better patient experiences by delivering self-administered options. Humira (and emerging biosimilars such as Amgevita) and Stelara, two top sellers, are conceived with the goal of serving patients through prefilled syringes. These two products alone grew in over $20bn in sales from 2015 to 2020, with the latter growing with a 30% CAGR in its prefilled syringe subsegment, quadrupling in value.
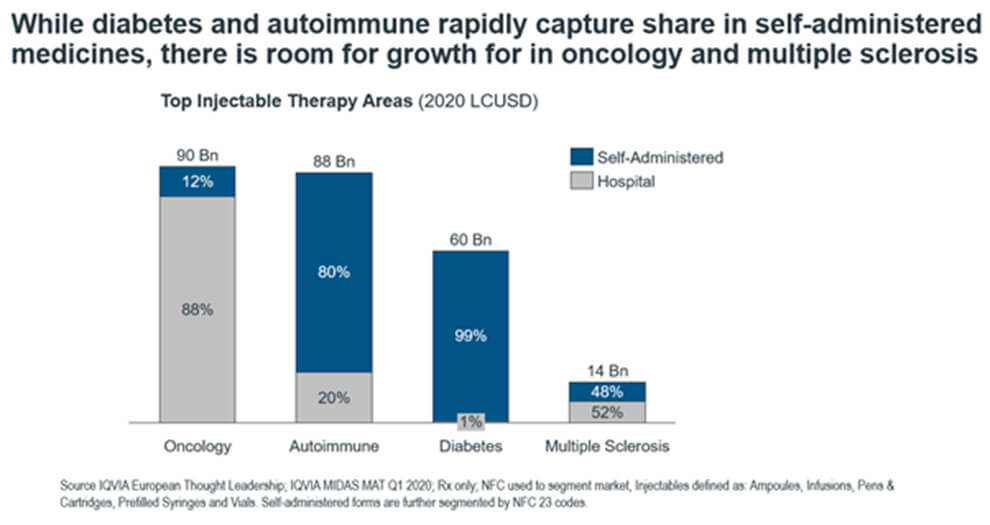
According to Figure 2, the segments that lag in growth are oncology and multiple sclerosis. While the Multiple Sclerosis (MS) segment saw the launch of blockbuster hospital-administered monoclonal antibody Ocrevus in 2017, which dominates the MS injectable share at nearly 30%, other drugs have not picked up pace in innovation toward self-administration. This is likely due to the anxiety associated with self-injection for patients with MS. The industry needs to dissuade patient fear in conjunction with developing formulations with learnings from high-growth segments such as autoimmune. This is already taking place, with the FDA approving Novartis’ Kesimpta for home-treatment in August 2020.
Figure 2. A view of potential growth for top therapy areas
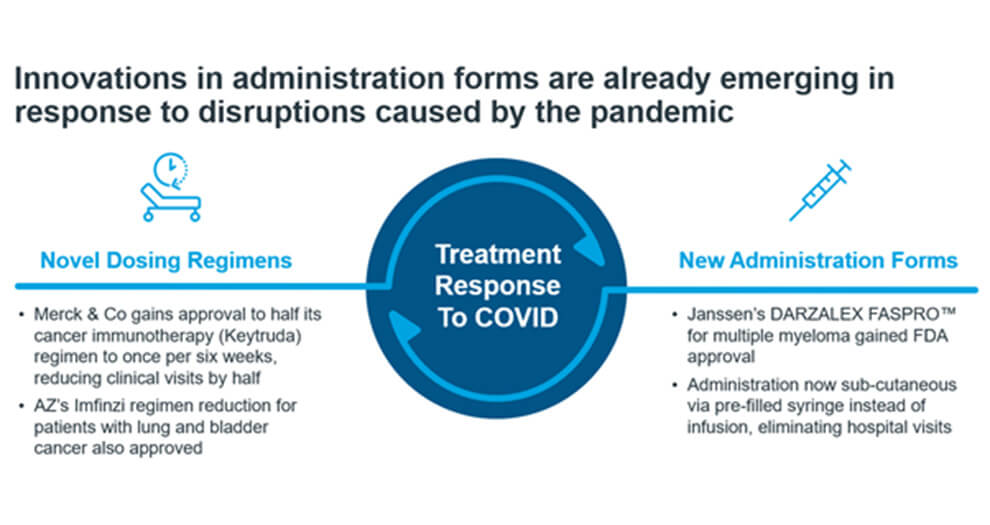
In oncology, treatments are typically administered at the hospital via infusion. However, outside pressure for change is growing stronger than ever, especially in light of the COVID-19 pandemic. With the decline of face-to-face visits, it is harder for patients to initiate or switch treatment options. Healthcare systems, like the NHS have pushed for interim cancer treatment recommendations that prioritise at-home-treatments for patients. As a result, the industry is already responding. Figure 3 shows innovations in dosing regimen and new formulations that aims to reduce hospital visits or time spent for drug administration. (1)
Figure 3. Innovations in formulation and dosing regimen in pandemic times
Overall, self-administered products lead growth in market share across multiple segments. With patients increasingly demanding more convenience for administration for chronic diseases, and with regulators pushing for less patient exposure to high-risk clinical settings during the pandemic, it seems the shift to self-administered drugs is inevitable. To future-proof against further waves of the pandemic, and to adapt to the potential collapse in the dynamic market, pharmaceutical companies need to ensure that better drug delivery systems and formulations is at the fore of their product development agenda.
For more information on trends in packaging and devices, please email me at [email protected]
References:

Related News
-
News Patients vs Pharma – who will the Inflation Reduction Act affect the most?
The Inflation Reduction Act brought in by the Biden administration in 2022 aims to give better and more equitable access to healthcare in the USA. However, pharma companies are now concerned about the other potential costs of such legislation. -
News CPHI Podcast Series: What does the changing US Pharma market mean for industry and patients alike?
In this week's episode of the CPHI Podcast Series Lucy Chard, Digital Editor for CPHI Online is joined by James Manser to discuss the political and market changes in the US pharma field. -
News CPHI Barcelona Annual Report illuminates industry trends for 2024
The CPHI Annual Survey comes into it’s 7th year to report on the predicted trends for 2024. Over 250 pharma executives were asked 35 questions, with their answers informing the industry landscape for the next year, spanning all major pharma marke... -
News Which 10 drugs are open to price negotiation with Medicare in the USA?
The Centres for Medicare & Medicaid Services, under the Biden administration in the USA, has released a list of the 10 drugs that will be open to price negotiations as part of the new legislation under the Inflation Reduction Act (IRA). -
News EU Medical Devices Regulation causes unintended disappearances of medical devices for children, doctors state
Doctor groups and associations have appealed to the EU to correct the EU Medical Devices Regulation law that may cause unintended shortages of essential drug and medical devices for children and rare disease patients. -
News 10 Major Drug Approvals So Far in 2023
Last year, 37 novel drugs were approved by the FDA, this was a high number for such a category, and covered many fields including oncology, demonstrating how promising further research is, and how it is only continuing to build. To date, there are alre... -
News Detecting Alzheimer's disease with a simple lateral flow test
A novel rapid diagnostic test for early-stage Alzheimer's disease has been developed using a biomarker binder from Aptamer Group along with technology from Neuro-Bio, the neurodegenerative disease experts. -
News CPHI Podcast Series: outsourcing and manufacturing trends
Listen to the CPHI Podcast Series this June to hear Gil Roth of the PBOA speak with Digital Editor Lucy Chard about the biggest trends and topics to watch in pharma outsourcing and manufacturing at the minute.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance