Keeping a close eye on processes and lines
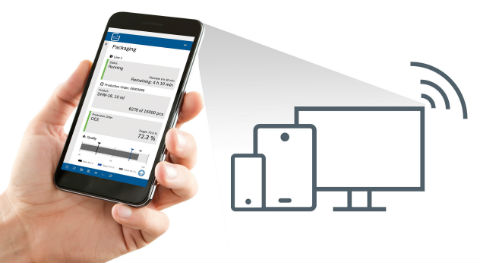
Real-time visualization and maximum flexibility.
New Industry 4.0 solutions from Bosch for more transparency.
High productivity and line availability are central priorities for pharmaceutical manufacturers. To provide more transparency and a better overview in production, Bosch showcases its latest Industry 4.0 solutions for visualizing and analysing machine data at Achema 2018.
Several machines and systems show how customers can use the Starter Edition of the Pharma Manufacturing Execution System (MES) to easily monitor machine status and process parameters. In addition, downstream Data Services make it possible to troubleshoot error causes more quickly, and to ensure more consistent product quality.
“Bosch’s Industry 4.0 solutions are tailored to the special requirements of the pharmaceutical industry and deliver full transparency for all process and machine data,” explains Dr Christian Hanisch, project manager Industry 4.0 for pharma at Bosch Packaging Technology. “The ability to record and evaluate essential machine and process data helps our customers maintain high machine availability and performance – and to make real-time decisions based on hard facts.” Bosch also shows how augmented reality specifically supports machine operators during commissioning or maintenance.
Real-time visualization with the Pharma MES Starter Edition
The Pharma MES Starter Edition with browser-based user interface consists of three main components: plant operators receive essential data on their overall equipment effectiveness (OEE), for condition monitoring of plant status or processes, and on important events like alarms or downtimes, simply and in real time. Visitors to Achema 2018 can experience the functions of the new Pharma MES Starter Edition firsthand on three machines: the new capsule filling machine GKF 720 and the new processing system SVP250 LF, combined with the filling and closing machine ALF 5000. Using a clearly structured and intuitive dashboard view on the respective machines, the system displays information on essential machine and production data, such as number of filled containers or OEE. The data is made available on the customer’s network via a web service and can be viewed on mobile devices or desktop PCs. This offers users maximum flexibility.
“Apart from individual machines, the Pharma MES Starter Edition can also monitor several lines simultaneously, and provides an important basis for optimum productivity,” Hanisch says. To do so, the system records information from various production units and stores it centrally. “To transfer data seamlessly, the Pharma MES Starter Edition is ideally calibrated for use with Bosch machines. However, with a suitable electronic interface, the solution can also be used with third-party production equipment,” he adds. The data gathered by the software can be subsequently analyzed to lay the groundwork for process optimizations, for instance, to enhance plant availability or performance. In addition, the Pharma MES Starter Edition provides operators access to historical data, allowing them to compare past and present data sets and gain valuable insights into the development of central parameters like OEE within a specific timeframe.
Data Services: identifying hidden correlations
Bosch also offers extensive data analysis services. The portfolio includes data mining, which Bosch has recently begun providing as part of the Pharma Service for solid dosage forms. With Bosch’s data mining tool, large amounts of data can be examined for the smallest effects using statistical methods. “Data mining makes it possible to assess information more effectively to identify and remedy the root causes of faults,” Hanisch says. In general, the data from two production batches is already sufficient to draw first conclusions. The more data is available for evaluation over a longer period, the more details will come to light.
Easy training thanks to AR technology
Bosch will demonstrate how commissioning and maintenance can be implemented more easily in future with its first augmented reality (AR) based instruction manual in the field of pharmaceutical development. Operators are guided step by step through the Solidlab 1 laboratory system, from setup to process control. Additional information, such as calibration instructions and spare parts are displayed so that employees can quickly work with the system without the need for extensive training. The AR instruction manual is suitable for training new colleagues or for refreshing knowledge. If there are any remaining questions, customers can use the remote function to connect directly with Bosch experts who provide live support.

Related News
-
News Pharmapack Awards 2024 Patient-Centric Design Award Winner – Dr Ferrer BioPharma
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024. -
News Women in Pharma: Minding the Gap at Pharmapack 2024
2024 marks the first year Pharmapack will host a Diversity track dedicated to bridging the gap within the pharmaceutical packaging and drug delivery sector. The track includes a panel discussion on 'Enabling Diversity in the Workplace,' focused... -
News Pharmapack Awards 2024 - Celebrating Packaging and Drug Delivery Innovation
The 2024 Pharmapack Innovation Awards ceremony celebrated the best in pharmaceutical packaging and drug delivery innovation at all levels. The awards were held on January 24, 2024 at the Paris Expo Porte de Versailles. -
News Pharmapack 2024 - From the Floor
Paris once again welcomes Europe’s leading trade show in pharmaceutical packaging and drug delivery innovation. Join our content team as Pharmapack 2024 opens its doors to leading experts and innovators in pharmaceutical packaging and drug delive... -
News CPHI Barcelona 2023: Partnering for Success – Managing Outsourcing Relationships to Optimise Manufacturing Operations
During CPHI Barcelona 2023, insightful content sessions offered attendees the chance to explore trending topics with expert speakers and panellists. Here, we summarise what the pharma industry and supply chain are talking about the most. -
News CPHI Barcelona 2023: Loading Potential – Artificial Intelligence for Pharma Manufacturing
During CPHI Barcelona 2023, insightful content sessions offered attendees the chance to explore trending topics with expert speakers and panellists. Here, we summarise what the pharma industry and supply chain are talking about the most. -
News Pharmaceutical industry supports COP28 health stance in joint statement
As COP28 takes place over this week in Dubai, UAE, several bodies in the pharmaceutical and health industries have come together to announce support of key movements in sustainability in the sector, and to recognise sustainability as a health issue.&nb... -
News CPHI Podcast Series: Start-ups take centre stage at CPHI Barcelona
The first episode of the CPHI Podcast Series since we attended CPHI Barcelona in October covers the Start-up market at the event, with expert Matthew Wise joining Editor Lucy Chard to discuss the event.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





.png)

