Key milestone in molded glass: SGD Pharma elevates Type I offering with three tailored product and service solutions
Strengthened value propositions represent the latest step in SGD Pharma’s commitment to continuous improvement
SGD Pharma, a leading supplier of glass primary packaging in the pharmaceutical industry and global expert in Type I molded glass, introduces
These market-driven value propositions represent the latest step in SGD Pharma’s ongoing improvements to its product offers and services. According to their specific business and parenteral drug requirements, Pharma and Biotech players would not only benefit from SGD Pharma’s enhanced high quality and reliable products, but also from an extended offer, ranging from Total Cost of Ownership (TCO) optimization to the highest levels of services.
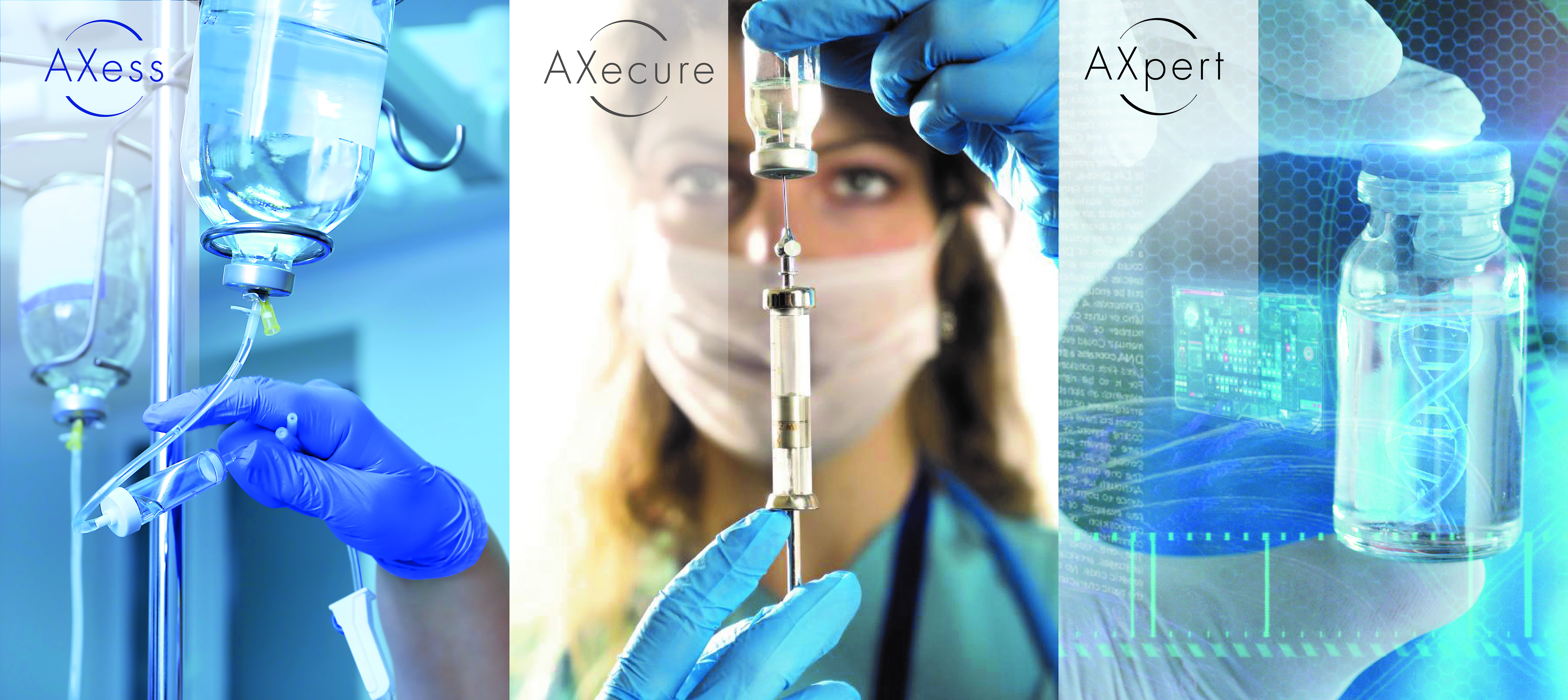
SGD Pharma has strategically organized its parenteral Type I molded glass offer into three tailored value propositions – AXess®, AXecure® and AXpert® – from which pharmaceutical manufacturers can easily choose, meeting their diverse drug product requirements and aseptic manufacturing constraints:
AXess delivers reliable, good quality glass packaging with straightforward TCO and is ideal for essential or primary care drugs that require inert Type I packaging. AXess delivers proven vial designs and optimized packaging configurations.
AXecure delivers built-in flexibility to cover all needs, with a wide range of superior quality vials for a diverse set of applications. AXecure is the standard for parenteral applications with an entirely customizable offering, from glass vial design to palletization options.
AXpert is designed for the most demanding and costly drugs that require the highest levels of quality and integrity, for applications such as oncology and specialty care. By providing access to advanced and personalized support and service at all times, AXpert protects your high value drug products, with total quality assurance and a range of premium value-added offerings.
SGD Pharma’s thorough analysis of parenteral market needs, including therapeutic applications, drug properties, and business environments, allows the company to meet the demand for high-quality glass vials that combine flexibility, quality assurance, and technical & regulatory support.
This strengthened packaging solution is the result of SGD Pharma’s continuous investments into its state-of-the-art plants, Saint Quentin-Lamotte (France) – a Type I glass Center of excellence – and Vemula (India), enabling the company to improve its products and services offering. With an output of 2.5 million vials per day, these best-in-class manufacturing facilities enable SGD Pharma to deliver these value propositions to all Pharma and Biotech customers, wherever they are located and regardless of supply chain needs.
Camille Ermine, Product Manager Parenteral at SGD Pharma, explains: “We are one of the largest and most experienced manufacturers of Type I molded glass globally, thanks to our purpose-built facilities. Manufacturing excellence is at the heart of the three-tier offering. With the development of the AXess, AXecure and AXpert ranges, this portfolio elevates SGD Pharma’s existing products and services, delivering additional levels of security, product choice, support, and global availability to support Business Continuity.”
To discuss which Type I molded glass range is best suited to your individual business needs, and to understand how molded glass can suit your packaging supply chain globally, please visit our website.

Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance