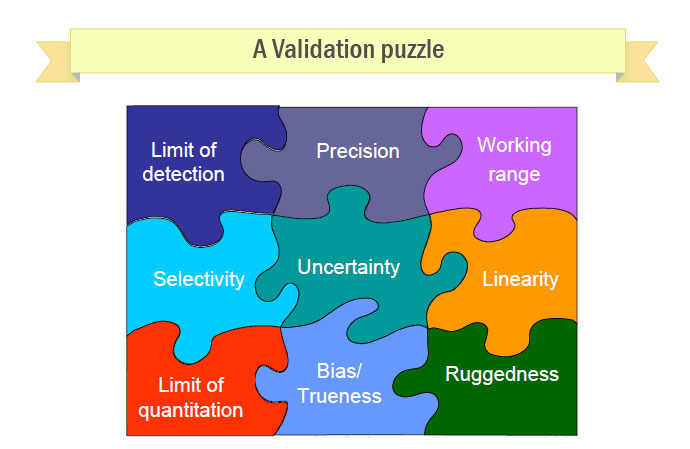
Method Development and Validation services
Product Description
Oasis Test House

-
IN
-
2018On CPHI since
Categories
Oasis Test House

-
IN
-
2018On CPHI since
More Products from Oasis Test House (2)
-
Product Microbiological Analysis
Oasis Test House caters to the microbiological requirements for its clients serving them with the below analysis. • Preservative Efficacy Analysis • Sterility Test • Sterility Validation • Microbial Limit Test Validation • Microbial count and pathogens • Bioburden testing • Antibiotics assay • Vitam... -
Product Stabiity Storage and Analysis
Stability studies for drug substances and products as per ICH Guidelines • Under investigation samples • Under approval samples • Developmental / market / production samples Assistance in protocol design, on-going sample testing and trend analysis during and at conclusion of stability testing
• Acc...
Oasis Test House resources (1)
-
Brochure Oasis Test House Brochure
Find more about our services.Oasis Test House - Your partner in Analytical services Techno-Capabilities Services · Batch Release and 2nd Testing · Analytical Method Development & Validation · Stability Storage and Analysis · Dissolution profiling studies Chemical & Instrumentation Analysis Amino Acid analysis Sugar analysis (Sucrose, Lactose, Mannitol, Sorbitol) In-Vitro Dissolution / Drug Release Studies for conventional and Modified Release Formulations Residual Solvents / Organic Volatile Impurities Particulate matter Elemental Analysis by AAS Microbiological Analysis · Preservative Efficacy Test · Antibiotics Assay · Vitamins Assay · Microbial count & Pathogens · Sterility Test · Bacterial Endotoxins Test (LAL test)
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance