Milla Pharmaceuticals Announces Approval and Launch of a Generic Version of Sodium Acetate Injection 2MEQ/mL by Pfizer Inc.
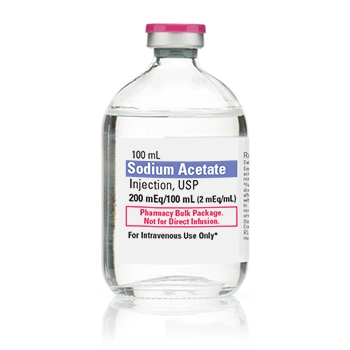
Sodium Acetate
MINNETONKA, Minn.--(BUSINESS WIRE)--Milla Pharmaceuticals, a subsidiary of the Alter Pharma Group, announced that it has received abbreviated new drug application (ANDA) approval from the U.S. Food and Drug Administration (FDA) for its generic version of Sodium Acetate Injection 2MEQ/mL.
Milla Pharmaceuticals was previously granted a Competitive Generic Therapy (CGT) designation for its generic drug by the FDA, which is indicated as a source of sodium for addition to large volume intravenous fluids to prevent or correct hyponatremia in patients with restricted or no oral intake. It is also used as an additive for preparing specific intravenous fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions.
As the first approved application, on July 7th, 2021, Milla Pharmaceuticals has established its first commercial sale through its exclusive partner, Woodward Pharma Services LLC, thus securing 180-days of market exclusivity. The FDA has recently listed Sodium Acetate Injection 2MEQ/mL on their FDA Drug Shortages List.
“Woodward Pharma Services LLC is excited to partner with Milla Pharmaceuticals to offer Sodium Acetate as part of Woodward’s growing hospital product portfolio,” said Nirav Patel, President of Woodward Pharma Services LLC.
This achievement marks a milestone for Milla Pharmaceuticals and the Alter Pharma Group not only as their first ANDA approval but also as a first-cycle review approval and garnering FDA’s coveted CGT Exclusivity. “After the launch by Sandoz of our generic injectable acetaminophen (paracetamol IV) in December 2020, being the first Alter Pharma product hitting the US market, this is another important step in our mission to make affordable and high-quality medicines available to all,” commented Filip Van de Vliet, CEO of the Alter Pharma Group, “thereby demonstrating the quite unique capabilities of our R&D and regulatory teams and partners to realize a first-cycle approval.”
Related News
-
News EU-GMP licence positions MediCane Health as possible cannabis export hub
MediCane Health's EU-GMP certification helps to bridge the pharma-cannabis gap -
News Panaxia's medical cannabis facility in Malta gains EU-GMP standard
This second facility gives the company a significant power multiplier for export capacity and geographical reach -
News China SXT's new lyophilisation facility secures pharmaceutical manufacturing permit
The move, coupled with the plants's passing of a pharmaceutical GMP compliance inspection, will enable company to accelerate the R&D of its novel Advanced TCMPs -
News Europe approves SK Bioscience’s global COVID-19 vaccine plant
CDMO's site becomes the first vaccine production facility in South Korea to obtain EU GMP certification, allowing it to ship to Europe -
News Pharma importers forced to reorganise supply chains due to Brexit complications, says medicines trade expert
EU/UK trade deal fell way short of UK’s initial expectations for medicinal products ahead of negotiations, says Peter Gough at NSF International -
News Pharma Explained: What is Quality Assurance?
Need clarity on determining your QA from your QbD and your cGMPs? You’re not alone! In our new Pharma Explained series from CPHI, we bring you clear cut definitions from industry experts on a myriad of Pharma Terms, delivered in bitesize video presenta... -
News 17th Annual CPHI Pharma Awards winners announced
Excellence in Pharma recognised at virtual ceremony taking place at the CPHI Festival of Pharma. -
News Successful MHRA regulatory inspection for Scottish CMO Symbiosis
Inspection conducted remotely using video-conferencing and an online private document sharing platform.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





1.jpg)

