Univercells introduces breakthrough vaccine manufacturing platform
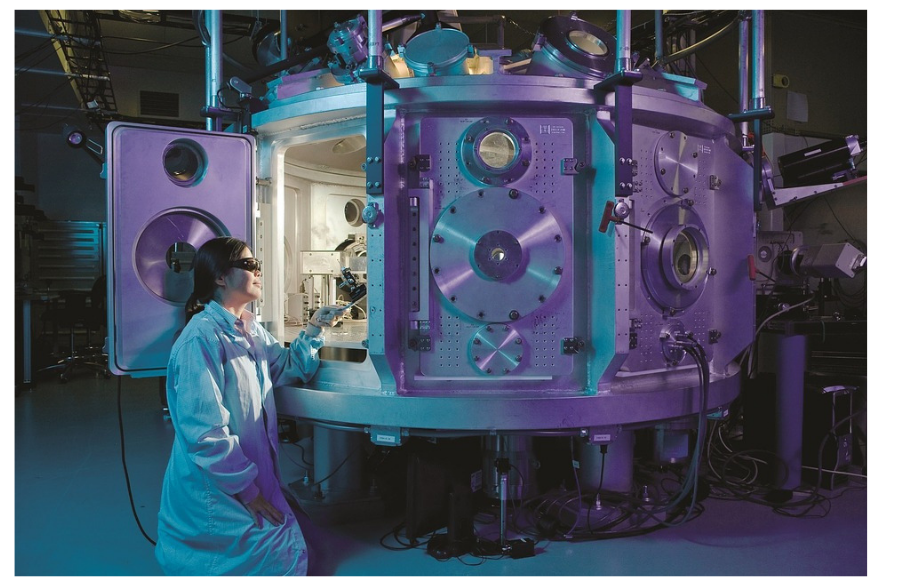
The automated NevoLine bioproduction system that facilitates safer, faster and closed bioprocessing in a much smaller footprint.
Univercells has launched its proprietary NevoLine bioproduction system for vaccines. The system was initially developed as part of a $12 million Grand Challenges grant awarded by the Bill & Melinda Gates Foundation to deliver affordable inactivated polio vaccine (sIPV). Leading the consortium, Univercells was responsible for the NevoLine system, Batavia Biosciences for the polio manufacturing process, and Merck for the purification membrane. After attaining its goals of delivering a very low Cost of Goods for an sIPV below $0.30 per trivalent dose, the consortium has now received a $4 million grant extension to scale-up the manufacturing system and process in preparation for clinical and commercial application. The first NevoLine system will be installed in Batavia Biosciences’ polio dedicated Biosafety level 3 Facility in Leiden, the Netherlands.
Based on a novel process architecture, Univercells designed the automated NevoLine bioproduction system that facilitates safer, faster and closed bioprocessing in a much smaller footprint. Through intensification and chaining of unit steps into a continuous process, users achieve high yields with less time and money invested. The sIPV production process using the NevoLine system is capable of producing trivalent sIPV at less than $0.30/dose, representing a five-fold reduction compared to current manufacturing technologies.
“This challenging 2-year project aimed at delivering a new manufacturing system to drastically decrease cost, footprint and time to market for vaccine manufacturers, and we are pleased to have met these goals,” Hugues Bultot, CEO and co-founder of Univercells said. “The NevoLine system is self-contained into a 6m² series of isolators. A facility designed with four NevoLine units would deliver up to 50 million sIPV doses per year for an estimated capital cost of $20 million. These breakthrough achievements further strengthen our dedication to innovating flexible, scalable and accessible vaccines and biotherapeutics manufacturing solutions.”
Dr Pierre A. Morgon, Non-Executive Director and Advisor to the CEO, added: “Since my first stint in the vaccine industry in 1986, I have seen few technological breakthroughs bringing a genuine opportunity to change the fundamentals of vaccine manufacturing. The technology developed by Univercells heralds a new era in vaccine production. The massive reduction of the upfront capital investment and of the manufacturing footprint required for bulk vaccine and biotherapeutics production will shake the markets to their foundations and enable greater competition by lowering barriers to entry. Public health stands to gain handsomely from these new market dynamics, with biotherapeutics and vaccines being available in large enough volumes and at affordable prices.”
Related News
-
News Pharmapack Awards 2024 Patient-Centric Design Award Winner – Dr Ferrer BioPharma
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024. -
News Women in Pharma: Minding the Gap at Pharmapack 2024
2024 marks the first year Pharmapack will host a Diversity track dedicated to bridging the gap within the pharmaceutical packaging and drug delivery sector. The track includes a panel discussion on 'Enabling Diversity in the Workplace,' focused... -
News Pharmapack Awards 2024 - Celebrating Packaging and Drug Delivery Innovation
The 2024 Pharmapack Innovation Awards ceremony celebrated the best in pharmaceutical packaging and drug delivery innovation at all levels. The awards were held on January 24, 2024 at the Paris Expo Porte de Versailles. -
News 2024 Pharma Industry Trends Outlook: Collaboration, Market Maturity, and Digital Futures
The annual CPHI Online 2024 Pharma Trends Outlook, in partnership with Arvato Systems, identifies 12 key industry trends shaping the life sciences industry in the coming year. -
News New Novo Nordisk AI hub for drug discovery to open in London, UK
Danish pharmaceutical giant Novo Nordisk will be opening an AI-based research facility in the heart of London to advance drug discovery operations. -
News BioNTech to begin mRNA vaccine manufacturing in Rwanda by 2025
German biotechnology company BioNTech has stated their intentions to begin production at their mRNA vaccine factory in Rwanda by 2025, which will mark the first foreign mRNA vaccine manufacturing site on the continent of Africa. -
News Women in Pharma: Looking back on 2023 and moving forward to 2024
In this monthly series, we interview women from across the pharmaceutical industry and supply chain to discuss the importance of gender diversity in healthcare, the workplace, and beyond. -
News CPHI Barcelona 2023: Partnering for Success – Managing Outsourcing Relationships to Optimise Manufacturing Operations
During CPHI Barcelona 2023, insightful content sessions offered attendees the chance to explore trending topics with expert speakers and panellists. Here, we summarise what the pharma industry and supply chain are talking about the most.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance







