Quality control
Quality control Companies (15)
Quality control News
-
News 2024 Pharma Industry Trends Outlook: Collaboration, Market Maturity, and Digital Futures
The annual CPHI Online 2024 Pharma Trends Outlook, in partnership with Arvato Systems, identifies 12 key industry trends shaping the life sciences industry in the coming year. -
News CPHI Barcelona Speaker Interview – What the US FDA’s Quality Management Maturity Means for the Pharma Industry
At CPHI Barcelona (24–26 October, 2023), we spoke to Sireesha Yadlapalli, CEO of Pharmatech Associates, who gave a presentation on the implications of the US FDA’s Quality Management Maturity (QMM) Initiative, and spoke on the panel of the ... -
News On track at CPHI Barcelona - The Track Sponsor interview: USP
In our packed out content sessions at CPHI Barcelona this year we focus on some of the hottest topics coming up in the pharma industry, with each track sponsored by a leading expert in the field. -
News Partnership between Scitara and Veeva to bring optimised QC lab productivity to customers
Scitara and Veeva have announced a partnership to bring improved data infrastructures to the quality control process of drug and therapeutic market release.
Quality control Products (79)
-
Product Sterile drug product CDMO services
Thermo Fisher Scientific's flexible aseptic manufacturing and sterile fill finish solutions for your molecule’s unique needs and challenges will enable success in early development, late-phase, and commercial manufacturing.
Thermo Fisher offers extensive sterile product development and commercial ...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Extractables and Leachables Testing Services
Testing for extractables and leachables: Our scientists perform extractables and leachables testing based on regionally specific guidelines (EU, US, China), nationally and internationally recognized standards (USP, ISO), GMP, PQRI recommendations, USP requirements (chapters <665>, <1663>, &l...
-
Product Qualified Person Release
As part of our wider service offering, our QPs can release clinical batches under our MHRA IMP license or fully licensed products under a commercial manufacturer/importer’s license. Manufacturing to GMP regulations, we support QP release to clinic, market or direct to you for onward pro...
-
Product Pantos Healthcare
Integrated International Transportation Solution: Each pharmaceutical product requires different transportation modes based on volume, value, lead time, and destination. Pantos Healthcare provides optimized transportation solutions based on the characteristics of pharmaceuticals, including air, ocean,...
-
Product Hi-End Resource On Demand Solutions
Project resource management includes forecasting the need to hire and/or outsource some tasks. When projects are high-value and require strategic decisions, in-house resources may be insufficient. Similarly, administrative action items are better left to contract workers so as to not overburden your staff....
-
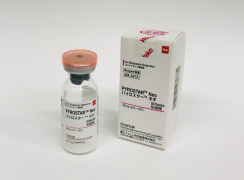
Product PYROSTAR™ Neo
PYROSTAR™ Neo is a new endotoxin detection reagent developed by recombinant technology.
PYROSTAR Neo is a lyophilized reagent that combines 3 recombinant factors (factor C, factor B, proclotting enzyme) with sequences derived from Limulus polyphemus and a chromogenic substrate (peptidyl pNA).The...
-
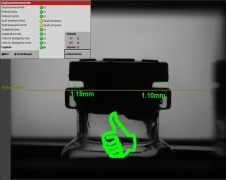
Product Container Closure Integrity Tester for laboratory use
With our wide range of lab testing equipment we provide solutions for the early stages in the development processes as well as for quality control in commercial manufacturing. Being able to transfer the test method from R&D to production reduces risks and saves time.
The NEO series combines high...
-
Product VialArch
The Gasporox sensor module VialArch offers non-destructive Headspace Gas Analysis to be integrated directly onto the production line or into an inspection machine.
This unique laser-based solution is installed on the production line for 100% quality inspection and container closure ...
-
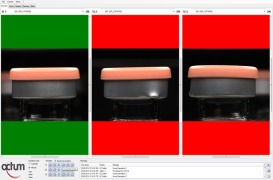
Product Visual Rotating Inspection machines
Inspection machines engineered to operate at high speeds on automatic production and packaging lines, designed to inspect a wide range of glass or plastic containers for liquids (such as solutions, suspensions, emulsions and oil-based products), powders or lyophilized products.
Our technologi...
-
Product PATVIS APA
Process Analytical Technology Visual Inspection System for Automated Particle Analysis. Sensum PATVIS APA is process analytical technology visualization system for monitoring, understanding and optimization of fluid bed pharmaceutical production processes. It enables real-time monitoring and fully automat...
-
Product Wasdell Laboratory Services
By the end of 2017 we will have a MHRA, GMP licenced laboratory. We will be offering a wide range of services for routine Quality Control analysis including QC release testing, method validation and stability analysis (analytical and microbiological). We work to the highest standards of GMP and GLP with r...
-
Product Ginkgo Biloba Extract
Changsha Huir Biological-Tech Co., Ltd.
is a Pre-listed professional herbal extracts and APIs manufacturer with Five Plants in China, Three Warehouses in USA(CA,NY,FL), One warehouse in Europe
Changsha Huir B...
-
Product SoftGroup® SaTT Site Controller
SoftGroup® SaTT Site Controller is a centralized serialization software system for the management of the serialization and aggregation processes. The Site management software by SoftGroup provides a complete solution for managing the whole production of the factory and preparing serialization and aggregati...
-
Product Quality Compliance
For Life Science businesses in highly regulated environments, compliance is not an option. Prepare to successfully pass inspections and design post-inspections remediation plans to achieve and maintain approval for your product with PQE Group's tailored and cost-effective programs for Quality Management.
-
Product Adenosine 5'-monophosphate, free acid (5'-AMP, free)
Yamasa corporation offers a wide range of biochemicals products which includes adenosine 5'-monophosphate, free acid (5'-amp, free). Contact us for more information.
-
Product Batch Release Services
PharOS has acquired a Certificate of GMP compliance from National Organization for Medicines for batch release of sterile and non-sterile products, as well as for imported medicinal products.
The PharOS team with its extensive experience offers to its clients batch release of their product...
-
Product CMC
Services include contract research and development and analysis for the purpose and regulatory dossier development as well as the corresponding registration applications.Production process studyRoute investigation and determination Laboratory & pilot levelKey starting materials and intermed...
Upcoming Events
-
CPHI & PMEC China 2024
Shanghai New International Expo Center
19 - 21 June 2024 -
CPHI South East Asia 2024
Queen Sirikit National Convention Center, Bangkok, Thailand
10 Jul 2024 - 12 Jul 2024 -
CPHI Korea 2024
COEX, Seoul, Korea
27 - 29 August 2024
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
















.png)

.png)


1.jpg)


.jpg)


.jpg)





.jpg)


















































































.png)



.png)



.jpg)