Aseptic Contract Manufacturing
Aseptic Contract Manufacturing Companies (4)
Aseptic Contract Manufacturing Products (11)
-
Product Outsourcing
Patheon by Thermo Fisher Scientific has a broad manufacturing platform for pharmaceutical and biologic products which provides sustainable solutions for mammalian cell-based and microbial-based manufacturing, green chemistry R&D and manufacturing technologies, and finished dosage production of biopharm...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Corporate Service Portfolio
We place heightened focus on your success as your strategic partner.
We produce aseptically prefilled syringes, cartridges, vials and dual-chamber systems as a globally operating CDMO partner. We are a family-owned, independent company rooted in 70+ years of history. We do not manufactu...
-
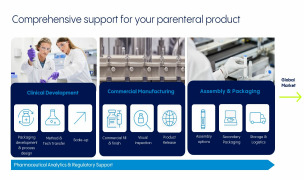
Product Contract Development and Manufacturing
We work closely with partners throughout their complete parenteral drug development program. As an FDA-approved CDMO, we ensure that the final process is scalable and successfully transfers to a cGMP–compliant commercial manufacturing process.
With decades producing high...
-
Product Sterile drug product CDMO services
Thermo Fisher Scientific's flexible aseptic manufacturing and sterile fill finish solutions for your molecule’s unique needs and challenges will enable success in early development, late-phase, and commercial manufacturing.
Thermo Fisher offers extensive sterile product development and commercial ...
-
Product Regulatory Support
Our Chemistry, Manufacturing and Controls (CMC) specialists help customers manage the product’s compliance at every step, from clinical development to market authorization. With decades of experience, our experts provide support in navigating regulatory milestones, including site registration, product ...
-
Product Syringe and Vial Filling for Clinical and Commerical
Lifecore’s experts have gained the know-how to build processes around your requirements for smooth scale-up and high-quality manufacturing.
Whether we’re your primary partner or a secondary source for manufacturing, we ensure you're fully satisfied with the final produ...
-
Product CDMO Biopharmaceutical Production
With an extensive offering of development services, state-of-the-art manufacturing equipment, and project support, GBI is truly a full-service CDMO biologics partner. Our approach pairs our expert knowledge base with an open mind towards scientific discovery so that together we can develop an optimal way t...
-
Product Filling of liquid formulations
Oncotec Pharma Produktion GmbH provides wide range of services which includes filling of liquid formulations. It is marketed as, among other things, sterile solutions for injection. It consist of a 150 l and 500 l batch and sterile container each. It supplies both the liquida and syringe filling machine. I...
-
Product DataCollector - High performance IT Infrastructure for Manufacturing
The plus10 DataCollector provides a high-performance data infrastructure for the acquisition and pre-processing of machine data from any source such as sensors, machine or robot controllers. Its simple configuration and implementation transforms machine data into Big Data and creates value through high-fre...
Upcoming Events
-
CPHI North America 2024
Pennsylvania Convention Center, Philadelphia
07 May 2024 - 09 May 2024 -
CPHI & PMEC China 2024
Shanghai New International Expo Center
19 - 21 June 2024 -
CPHI South East Asia 2024
Queen Sirikit National Convention Center, Bangkok, Thailand
10 Jul 2024 - 12 Jul 2024
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance








.jpg)





.png)


.png)



.jpg)