Medical and Pharmaceutical Industry Technology and Development Center
About Medical and Pharmaceutical Industry Technology and Development Center
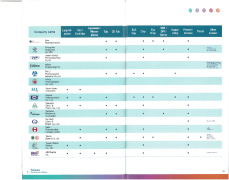
Categories
-
TW
-
2018On CPHI since
-
100 - 249Employees
Company types
Meet us at
CPHI Japan 2024
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
17 Apr 2024 – 19 Apr 2024
Medical and Pharmaceutical Industry Technology and Development Center Resources (1)
-
Brochure TAIWAN PHARMACEUTICAL ALLIANCE 2023 Brochure
TAIWAN PHARMACEUTICAL ALLIANCE RESOURCES
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance