Biapenem(GMP)

Product Description
SIMCERE PHARMACEUTICAL GROUP

-
CN
-
2015On CPHI since
Specifications
SIMCERE PHARMACEUTICAL GROUP

-
CN
-
2015On CPHI since
More Products from SIMCERE PHARMACEUTICAL GROUP (2)
-
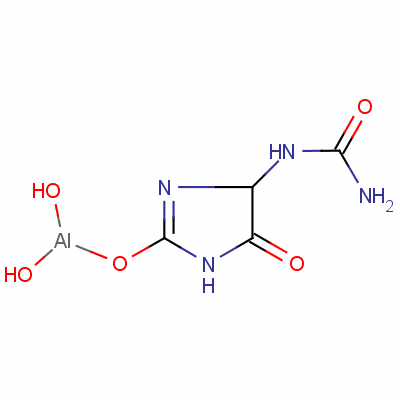
Product Aldioxa
Aldioxa
CAS:5579-81-7
Introduction:
Aldioxa is a condensation product of allantoin and aluminum hydroxide. Its formula is C3H7AIN4O5. It is present in multi-ingredient preparations intended for treating gastric and duodenal ulcers, chronic gastritis, gastric inflammation, drug-induced gastritis. I... -
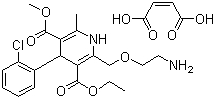
Product Amlodipine Maleate (GMP)
Amlodipine Maleate
CAS:88150-47-4
Introduction:
Amlodipine Maleate is a calcium channel blocking agent,and It is effective in the treatment of angina pectoris and hypertension. It inhibits the influx of extracellular calcium across the myocardial and vascular smooth muscle cell membranes. The decr...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance