First prostate cancer patients treated with Augmenix's SpaceOAR Hydrogel in Israel
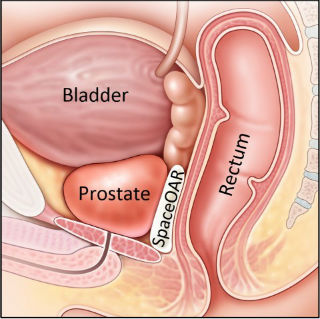
SpaceOAR Hydrogel separates the prostate and rectum during radiation treatment (Photo: Business Wire).
The Chaim Sheba Medical Center now offers the hydrogel spacer to reduce side-effects during prostate cancer radiation therapy.
Augmenix has announced that the first patients have been treated with SpaceOAR hydrogel at the Chaim Sheba Medical Center in Tel Hashomer, Israel. SpaceOAR hydrogel is an absorbable prostate-rectum spacer that reduces rectal injury during prostate radiotherapy.
“We are excited to offer our patients this new, innovative technology to significantly reduce risks of prostate cancer radiotherapy,” said Zvi Symon, from the Chaim Sheba Medical Center. “SpaceOAR hydrogel will provide meaningful long-term benefits, improving overall quality of life for our patients."
“We are extremely pleased to bring our innovative SpaceOAR hydrogel to physicians and patients in Israel," commented John Pedersen, CEO of Augmenix. "Our growing body of clinical evidence demonstrates that SpaceOAR hydrogel significantly reduces the risk of rectal and urinary toxicities and loss of sexual function associated with prostate cancer radiotherapy. SpaceOAR hydrogel is making a significant difference in the lives of men around the world.”
Radiation therapy in the treatment of prostate cancer can cause unintended injury to adjacent healthy tissue, which can lead to bowel, urinary and sexual symptoms that can affect patient health and quality of life. With SpaceOAR hydrogel, physicians can place a hydrogel barrier to separate the prostate from surrounding healthy tissue.
In January 2017, Augmenix announced 3-year post-treatment data from a prospective, randomized, multi-center, patient-blinded clinical trial showing that patients treated with SpaceOAR hydrogel technology prior to prostate cancer radiotherapy demonstrated significant rectal (bowel), urinary, and sexual benefit through three years of follow up. Overall patient wellness at 3 years was assessed by looking at the percent of patients with clinically significant declines in all three quality of life (QOL) domains (bowel, urinary and sexual). Fully 20% of men in the Control arm had clinically significant declines in all three QOL areas compared to only 2.5% of men in the SpaceOAR hydrogel arm. Among men who were potent at baseline, the analysis showed that SpaceOAR hydrogel treated men were better able to maintain erections sufficient for intercourse through 3 years of follow-up. Of the men treated with SpaceOAR hydrogel, 66.7% could achieve erections sufficient for intercourse at 3 years compared with 37.5% in the Control arm, a 77.8% improvement.
Related News
-
News US BIOSECURE Act passed by US House of Representatives
The controversial act, which has already impacted several foreign companies operating in the US, was passed by the House of Representatives on September 9, 2024. It is now headed for the US Senate before it can be signed into law by President Joe Biden... -
News Pharma Supply Chain People Moves
The latest appointments, promotions, and structural changes across the pharmaceutical supply chain. -
News Drug prices agreed upon as part of the US Inflation Reduction Act
The Inflation Reduction Act brought into constitution by the Biden administation in 2022, which proposed a drug price negotiation between the government and pharmaceutical companies, has reached it's first agreement. -
News BIOSECURE Act continues to loom over Chinese pharma manufacturers
With the US BIOSECURE Act on its way to passing into legislation, Chinese companies are facing declining revenues within the first half of 2024 as US pharmaceutical and healthcare companies pull their businesses from the country. -
News Ophthalmologic drug product Eylea faces biosimilar threats after FDA approvals
Regeneron Pharmaceutical’s blockbuster ophthalmology drug Eylea is facing biosimilar competition as the US FDA approves Biocon’s Yesafili and Samsung Bioepis/Biogen’s Opuviz. -
News ONO Pharmaceutical expands oncology portfolio with acquisition of Deciphera
ONO Pharmaceutical, out of Japan, is in the process of acquiring cancer-therapy maker Deciphera Pharmaceuticals for US$2.4 billion. -
News First offers for pharma from Medicare drug price negotiations
Ten high-cost drugs from various pharma manufacturers are in pricing negotiations in a first-ever for the US Medicare program. President Biden’s administration stated they have responded to the first round of offers. -
News Eli Lilly’s Zepbound makes leaps and bounds in weight-loss drug market
In the last week, Eli Lilly has announced their partnership with Amazon.com’s pharmacy unit to deliver prescriptions of Zepbound. Zepbound has also surpassed Novo Nordisk’s Wegovy for the number of prescriptions for the week of March 8.&nbs...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance



.png)
.png)

.png)
.png)