CuraTeQ Biologics
About CuraTeQ Biologics
Categories

-
IN
-
2024On CPHI since
-
250 - 499Employees
Company types
Primary activities
Meet us at
CPHI Milan 2024
Fiera Milano, Italy
08 Oct 2024 - 10 Oct 2024
Products from CuraTeQ Biologics (2)
-
Product Biosimilars Portfolio
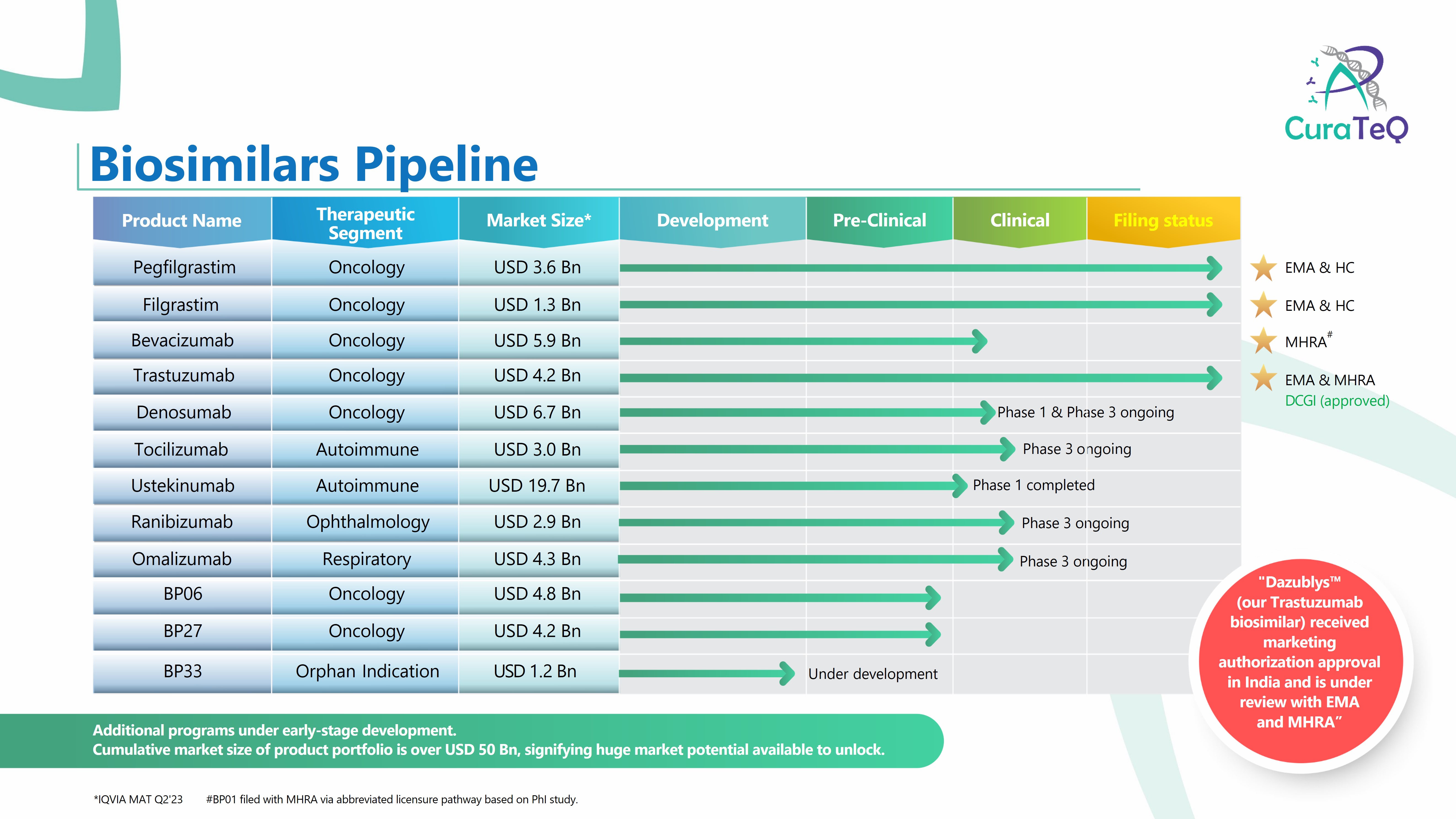
• Pegfilgrastim (Filed with EMA) • Filgrastim (Filed with EMA) • Trastuzumab (Filed with EMA, MHRA, Received recommendation for marketing authorization in India) • Bevacizumab (Filed with MHRA) • Omalizumab (Global PhIII ongoing) • Denosumab (Global PhIII ongoing) • Ranibizumab (Global PhIII ongoing... -
Product Synthetic Peptide APIs Portfolio
• Vasopressin (US DMF Approved) • Icatibant acetate (US DMF Approved) • Leuprolide acetate (US DMF Approved) • Linaclotide (US DMF Approved) • Etelcalcetide (US DMF Approved) • Desmopressin (US DMF Approved) • Calcitonin salmon (US DMF Filed) • Ganirelix acetate (US DMF Filed) • Degarelix (US DMF ...
CuraTeQ Biologics Resources (1)
-
Brochure Synthetic Peptide APIs Portfolio
Auro Peptides, our synthetic peptides arm, has filed 14 US DMFs which have contributed to 6 ANDA approvals.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance