Audit
Audit Companies (15)
Audit News
-
News Mitigating supply risk: Having feet on the ground during COVID-19
Having personnel on the ground may be central to mitigating supply chain risks, even as the world embraces the remote approach. -
Sponsored Content Audits in the time of COVID-19 – Implications for a CMO
Travel restrictions imposed by the pandemic have limited the ability of pharmaceutical companies and health authority personnel to travel and conduct in-person inspections of contract manufacturing organizations (CMOs). Due to t... -
News GVK BIO Announces USFDA Approval for its cGMP Analytical Services Laboratory
GVK BIO, a leading global Contract Research and Development Organisation, announced today that United States Food and Drug Administration (FDA) has approved its cGMP Analytical Service Laboratory located at IDA, Mallapur Campus, Hyderabad.
Audit Products (39)
-
Product GxP Audits and Inspection Readiness
Our auditors have real world experience supporting regulatory inspections and preparing companies for pre-approval inspections. Our team of specialists includes former regulatory agency inspectors and qualified auditors who are proficient in conducting mock inspections, internal audits, vendor and s...
-
Product AI and non-AI validation technologies for Life Science companies
We specialize in IA and non-AI Computer System Validation, equipment validation, utilities qualification, and IT/OT infrastructure qualification. Our services comply with the regulatory standards of the EMA, FDA, WHO, and other key Life Science authorities across Europe and the Americas. With a proven trac...
-
Product Pharmaceutical Auditing Services
Auditing services for the pharmaceutical industry: Our pharmaceutical audits are conducted in accordance with the relevant regulatory texts or standards, including GMP, GDP, GLP, GCP, and GVP. We also audit against IPEC guidelines for Pharmaceutical excipients, ISO 22716 (Cosmetics — Good Manufactu...
-
Product OC11 Word Platform
The OC 11 Platform is not just another software; it's the next generation of Global GxP and Data Integrity solutions. It's designed to revolutionize how GxP records are generated and to ensure users comply with global GxP and Data Integrity regulatory requirements..
Convert any Word form i...
-
Product GMP / QP Audits
PharmSol carries out a complete chain of audits which assures product safety and quality to clients across the globe for Finished Products, APIs, Intermediates and Key Starting Materials (KSMs). PharmSol has an in-house team of Auditors who are accredited with various certifications including APIC/CEFIC/AS...
-
Product Validation
Our team of experts will prepare necessary protocols incorporating all critical parameters
Process Validation:
PharmEng professionals possess experience in validating pharmaceutical and biopharmaceutical manufacturing processes. Our team of experts will prepare necessary protocols i...
-
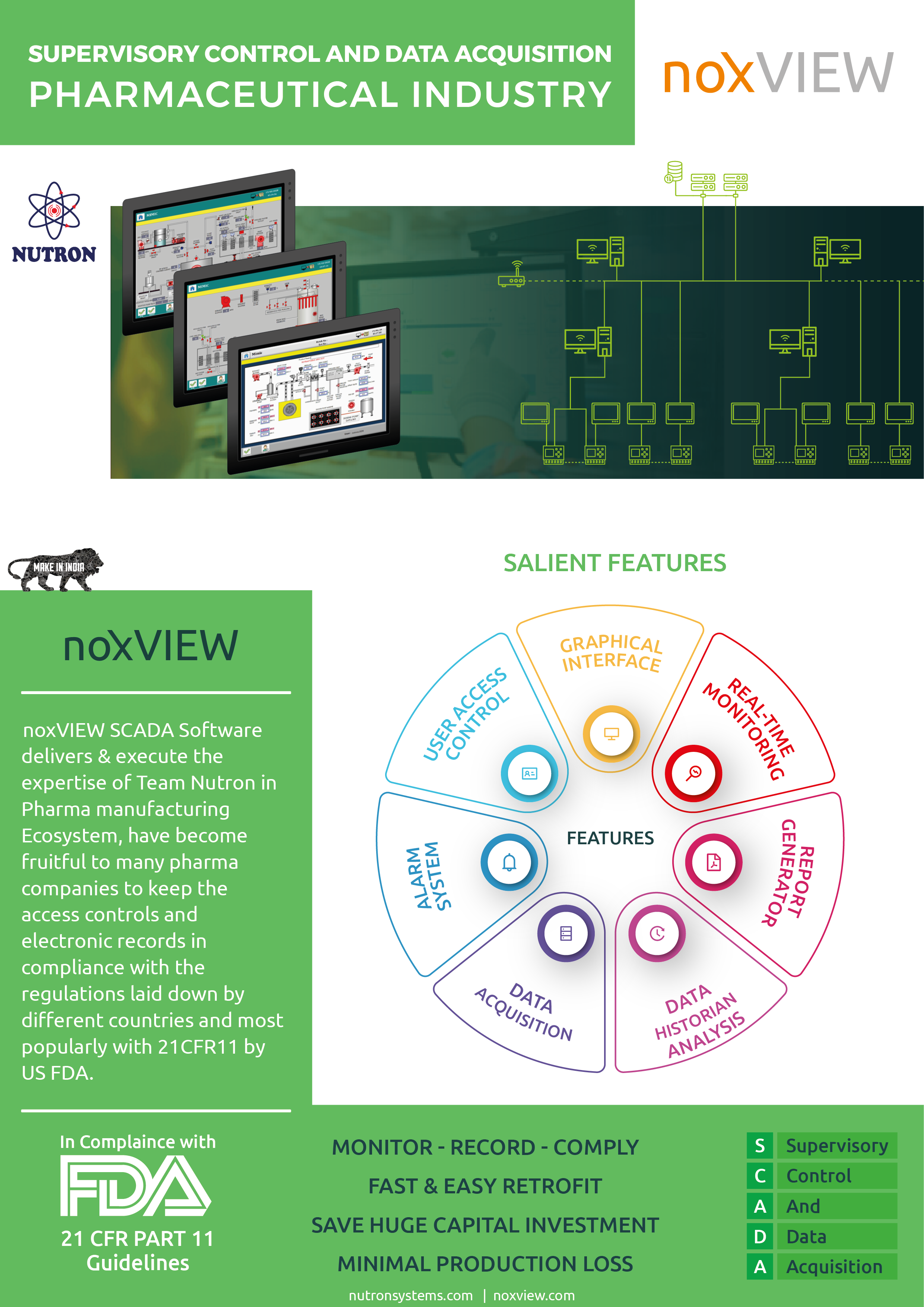
Product 21CFR SCADA ( Centralized Monitoring & Control ) - Software Solution
A hardware independent SCADA software provides limitless analytics and reporting needs with built in historian. Very Popular for 21CFR11 Compliance in Pharma industry
-
Product Audits
Due to recent regulatory developments concerning raw materials, suppliers, manufacturing and distribution, auditing has become a task of critical importance for Life Science businesses.PQE Group supports Pharma and MD companies in performing many types of certified audits, from routine monitoring to due di...
-
Product Computerized System Validation (CSV) and Compliance Service
CSV implementation, Data Integrity, 21 CFR Part 11 & Eu Annex 11 Compliance Assessment, Implementation of Lab Applications like Electronic Lab Notebook, LIMS, SDMS, CDS, etc.,
GxP - Third party audits, Validation, Qualification and Engineering services, Remediation projects, Quality, Training &a...
-
Product Quality & Compliance Solutions
From early-stage development through commercial support, our experts provide critical GxP-based consulting services. We partner with clients to successfully execute projects throughout the product lifecycle to maintain quality & compliance with applicable regulations and industry standards. qq...
-
Product EU/UK Qualified Person (QP) Services and MIA license
Navigating both general and country-specific regulations and requirements to supply medicinal products to the European markets can be a complex challenge for Marketing Authorization Holders (MAH). ProPharma holds both EU and UK MIA licenses which allows us to help clients overcome the complexities of su...
-
Product GO!FIVE Digital Validation Solution
Global Verification & Validation is a SaaS solution for Life Science companies that complies with EMA/FDA. FIVE Validation developed a SaaS platform that allows AI and traditional validation studies to proceed 6x faster than a manual (electronic or paper-based) process.
-
Product Compliance Management
PharmSol does not limit itself only to offering Audits, but we expand our solution offerings to ensure complete Compliance at the client’s site. Towards obtaining GMP Certification from Europe, United States or WHO, PharmSol has a very effective, systematic and rational approach in providing solutions effi...
-
Product USFDA Compliance & Regulatory
Preparing for a USFDA Inspection and managing the post inspection situation requires a high level of expertise and PharmSol offers a comprehensive solution in that direction.-Compliance Management
-Product Development
-Regulatory Support
-BD Support
-
Product Regulatory Affairs
We provide solutions to meet both local and global regulatory requirements in the most cost-effective manner.
PharmEng supports Pharmaceuticals, Biologics, Over-The-Counter (OTC), Animal Health, Radiopharmaceuticals, Clinical Trial Products, Medical Devices, Combination Products, Natur...
-
Product Project Management
Our experts incorporate the most modern and efficient Project Management techniques to ensure all project goals and objectives are met
PharmEng Technology has recognized that Project Management is essential throughout the Pharmaceutical, Biotechnology, and Medical Device industries.
...
-
Product Engineering
We help bring concept ideas to life
PharmEng engineers are experienced in building scalable and robust pharmaceutical production facilities and processes while employing state of the art techniques to meet and exceed operational and regulatory requirements.
Our experts have succes...
-
Product Commissioning & Qualification
Our qualified consultants strive to exceed your project goals and objectives
Our flexibility and project experiences range from the qualification of an individual piece of equipment to an entire facility.
Our PharmEng professionals have extensive experience in the development, imple...
-
Product Quality Systems
We establish and manage quality systems specifically tailored to clients’ operational needs.
Our clients understand the importance of establishing a robust quality system; The effort in maintaining a strong quality system not only ensures that the products and services meet the customer expectat...
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

























































.png)







.png)

.png)



.jpg)


