BD
About BD
Categories

-
US
-
2017On CPHI since
Products from BD (3)
-
Product BD Vystra™ Disposable Pen
Discover our portfolio of solutions and services for a wide variety of therapeutic areas and settings: from home to hospital through outpatient clinic, and from vaccines to chronic disease treatments through small molecules and specialties, dermal fillers and contrast media… We provide unparalleled parente... -
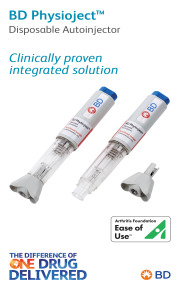
Product BD Physioject™ Disposable Autoinjector
Discover our portfolio of solutions and services for a wide variety of therapeutic areas and settings: from home to hospital through outpatient clinic, and from vaccines to chronic disease treatments through small molecules and specialties, dermal fillers and contrast media… We provide unparalleled parente... -
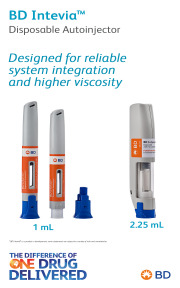
Product BD Intevia™ Disposable Autoinjector
Discover our portfolio of solutions and services for a wide variety of therapeutic areas and settings: from home to hospital through outpatient clinic, and from vaccines to chronic disease treatments through small molecules and specialties, dermal fillers and contrast media… We provide unparalleled parente...
BD Resources (2)
-
News Pharmapack 2024 - From the Floor
Paris once again welcomes Europe’s leading trade show in pharmaceutical packaging and drug delivery innovation. Join our content team as Pharmapack 2024 opens its doors to leading experts and innovators in pharmaceutical packaging and drug delivery. -
Brochure BD Product Portfolio
Discover our portfolio of solutions and services for a wide variety of therapeutic areas and settings: from home to hospital through outpatient clinic, and from vaccines to chronic disease treatments through small molecules and specialties, dermal fillers and contrast media… We provide unparalleled parenteral drug delivery systems, including glass and plastic syringe systems, self-injection devices, safety and shielding systems that set the standard.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance