BD Neopak™ XtraFlow™ Glass Prefillable Syringe for Biotech - Enabling the delivery of complex and viscous biologics
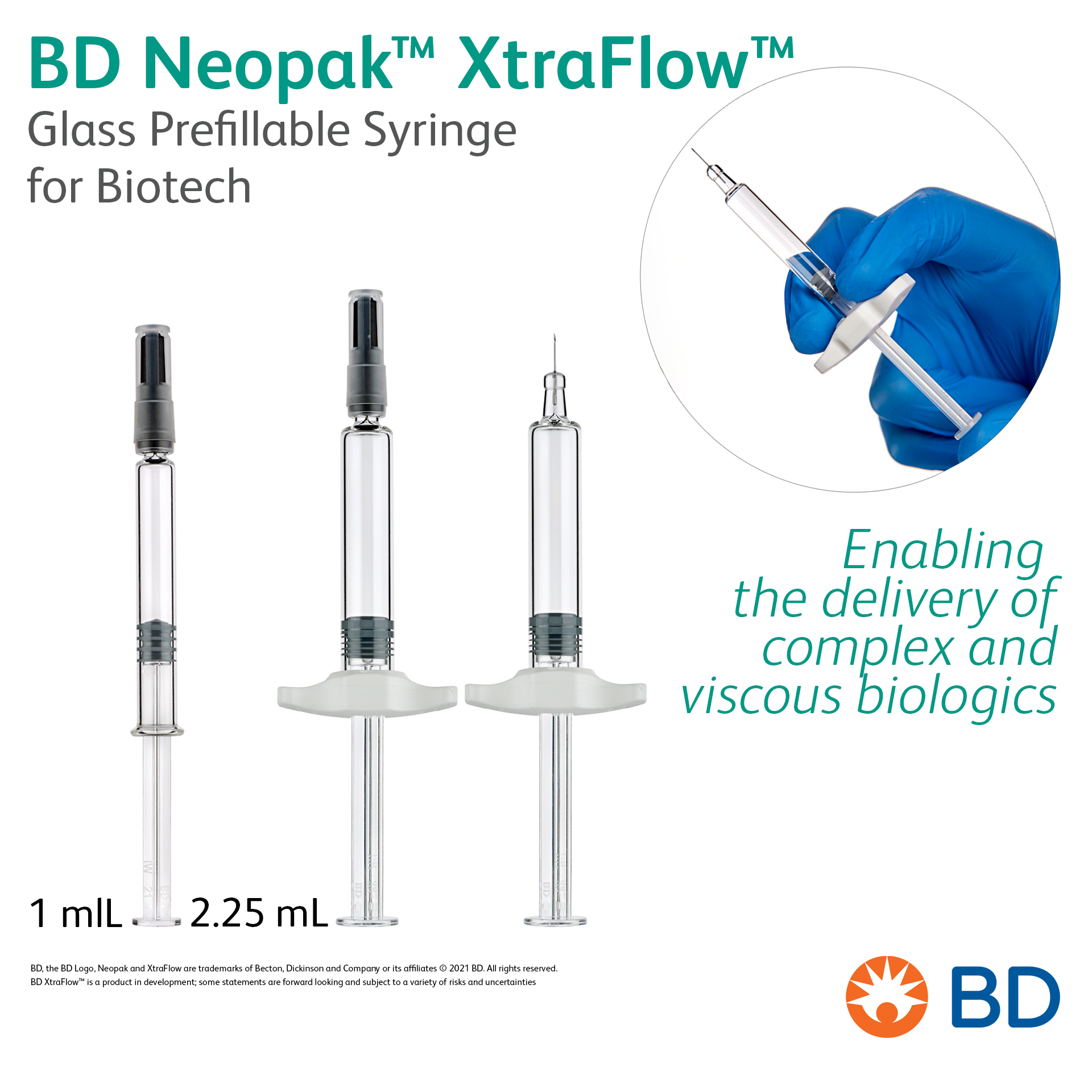
Product Description
SAS BECTON DICKINSON FRANCE (BD)

-
FR
-
2015On CPHI since
-
2Certificates
-
5000+Employees

Company types
Primary activities
Categories
SAS BECTON DICKINSON FRANCE (BD)

-
FR
-
2015On CPHI since
-
2Certificates
-
5000+Employees

Company types
Primary activities
More Products from SAS BECTON DICKINSON FRANCE (BD) (25)
-
Product Medication Delivery Solutions - Product Portfolio
Solutions for reconstitution, injection and infusion…
BD Medical - Medication Delivery Solutions serves your varied needs for commercial drug co-packing, co-delivery or clinical trial material.
Contact: BD Medical - Medication Delivery Solutions (Europe) - Route De Crass... -
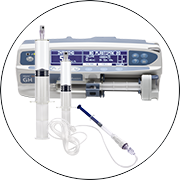
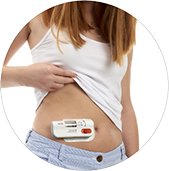
Product BD Libertas™ Wearable Injector - Supporting subQ delivery of large volume complex biologics
BD Libertas™ Wearable Injector is an innovative, hands-free injection system designed to automatically deliver high volume and/or highly viscous biotech drugs subcutaneously. It delivers a wide range of therapeutic drugs to the subcutaneous tissue over a period of time, ranging from a few seconds to s... -
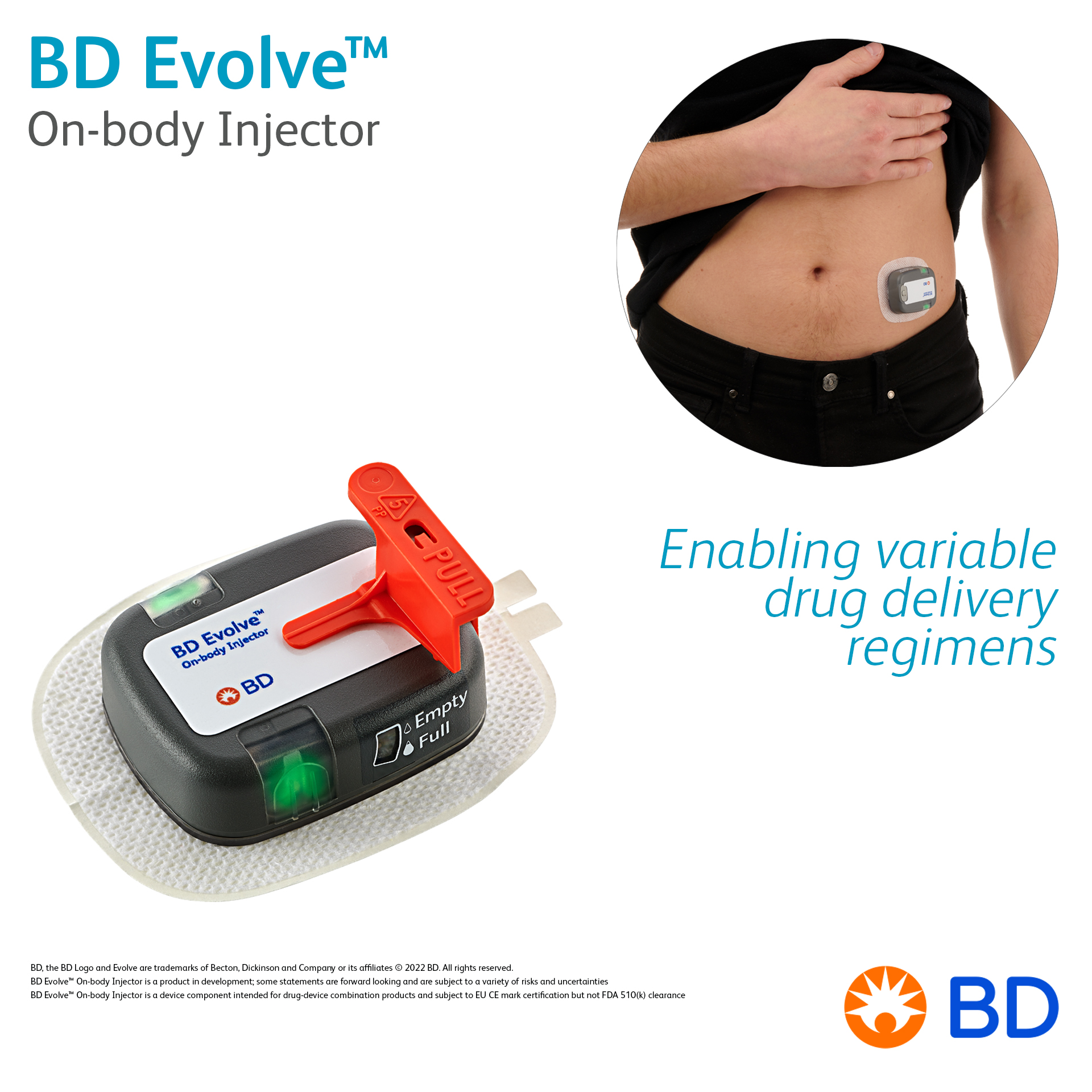
Product BD Evolve™ On-body Injector - Enabling variable drug delivery regimens
BD Evolve™ On-body Injector is being developed for pharmaceutical companies who seek flexible delivery options, as well as opportunities for improving the patient experience and lowering the cost of care. It supports delivery of biologics with customizable and programmable subcutaneous administration.... -
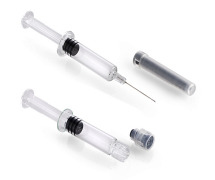
Product BD Hylok™ Glass Prefillable Syringe For Hyaluronic Acid - Connection performance for HA injections validated in Human Factor studies
BD Hylok™ Glass Prefillable Syringe for Hyaluronic Acid is a new luer syringe for the administration of viscous drugs. It offers customers the advantages of glass containers (e. g. medication stability) with a robust luer connection. The first targeted application for BD Hylok™ is hyaluronic acid... -
Product BD Physioject™ Disposable Autoinjector - Designed to support the treatment of chronic diseases with biologics
BD Physioject™ Disposable Autoinjector is designed to fully integrate with BD Neopak™ and BD Hypak™ for Biotech 1 mL long Glass Prefillable Syringes to ensure optimal system functionality.
BD partners with you to develop self-injection systems that enable drug administration ac... -
Product BD Vystra™ Disposable Pen - Confidence in dose delivery
BD Vystra™ Disposable Pen is an intuitive, high-quality and customizable pen injector designed to support therapies that require frequent, low-volume injections or variable dosing.
BD partners with you to develop self-injection systems that enable drug administration ac... -
Product BD Neopak™ XSi™ Glass Prefillable Syringe for Biotech - Addressing silicone-related concerns
BD Neopak™ XSi™ Glass Prefillable Syringe For Biotech combines BD Neopak™ Syringe with proprietary immobilized silicone coating, significantly reducing potential concerns associated with silicone without introducing new chemical substances.
BD offers a broad portfolio of prefillable ... -
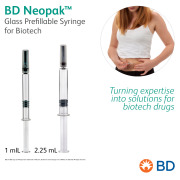
Product BD Neopak™ Glass Prefillable Syringe for Biotech - Turning expertise into solutions for biotech drugs
BD Neopak™ Glass Prefillable Syringe For Biotech features advanced technologies to meet the highest expectations of leading biopharmaceutical companies. BD Neopak™ is available in both 1 mL and 2.25 mL formats.
BD offers a broad portfolio of prefi... -
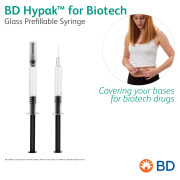
Product BD Hypak™ for Biotech Glass Prefillable Syringe - Covering your bases for biotech drugs
BD Hypak™ for Biotech is our versatile prefillable syringe delivery system that addresses key factors of compatibility with biopharmaceutical drugs.
BD offers a broad portfolio of prefillable solutions specifically designed for biopharmaceuticals and chronic disease treatmen... -
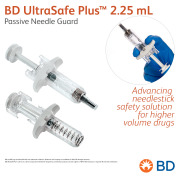
Product BD UltraSafe Plus™ 2.25 mL Passive Needle Guard - Advancing needlestick safety solution for higher volume drugs
BD UltraSafe Plus™ Passive Needle Guard for prefilled ISO standard 1 mL long and 2.25 mL glass syringes is designed to support viscous drug delivery with ergonomics and drug visibility.
BD offers a broad portfolio of prefillable solutions specifically designed for biopharmaceutica... -
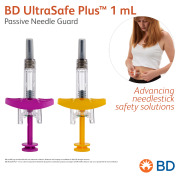
Product BD UltraSafe Plus™ 1 mL Passive Needle Guard - Advancing needlestick safety solutions
BD UltraSafe Plus™ Passive Needle Guard for prefilled ISO standard 1 mL long and 2.25 mL glass syringes is designed to support viscous drug delivery with ergonomics and drug visibility.
BD offers a broad portfolio of prefillable solutions specifically designed for biopharmaceutica... -
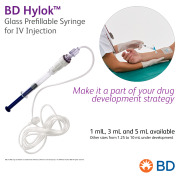
Product BD Hylok™ Glass Prefillable Syringe For IV Injection - Make it a part of your drug development strategy
BD Hylok™ Glass Prefillable Syringe for IV Injection is a new luer syringe for the administration of IV drugs. It offers customers the advantages of glass containers (e. g., medication stability) with a robust luer connection. BD Hylok™ is a ready-to-use glass solution, c...
SAS BECTON DICKINSON FRANCE (BD) resources (75)
-
News BD Completes Study Investigating Performance of Glass Prefillable Syringes (PFS) in Deep Cold Storage
Study reveals glass PFS can be stored at -20°C and -40°C without risk on PFS key product functions, establishing pathway to further assess the stability and performance of drug combination products with PFS in deep cold conditions. -
Brochure BD SCF™ PremiumCoat™ 1 mlL Plunger Stopper - Supporting the delivery of biologics in 1 mlL prefilled systems
Discover BD SCF™ PremiumCoat™ 1 mlL Plunger Stopper designed to serve the evolving needs of pharmaceutical organizations in the development of combination products for chronic diseases.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo, Intevia, Neopak, SCF, UltraSafe Plus, Visioguard and XSi are trademarks of Becton, Dickinson and Company or its affiliates. All other trademarks are the property of their respective owners. © 2021 BD. All rights reserved.
-
News BD unveils $1.2 billion investment in pre-fillable syringe manufacturing
World’s largest syringe maker gears up for increased demand across all product classes amid expected COVID-19 vaccine roll out -
Brochure How BD offers support to COVID-19 drug and vaccine developers
In this interview published in June 2020 in a special COVID-19 issue of Pharma’Post, Marie-Liesse Le Corfec, Head of Global Portfolio Marketing, presents the variety of means and capabilities deployed by BD to support Covid-19 drug and vaccine developers, help accelerate time-to-market and support global health.
https://drugdeliverysystems.bd.com/contact-us
BD and the BD Logo are trademarks of Becton, Dickinson and Company © 2021 BD. All rights reserved. -
News Vaccine development toolkit needs to be optimized to counter infectious disease threat: CPHI webinar
While there have been some huge advances in vaccine technology, more still needs to be done to counter the threat that infectious diseases pose, particularly to poorer countries. -
Brochure BD Services & Solutions - Develop. De-risk. Deliver. [Brochure]
Discover a comprehensive set of services and solutions designed to mitigate the risks associated with combination product development to help you achieve your goals. These services and solutions help you from development all the way through to launch, including container selection, combination product expertise and testing, regulatory and technical documentation support, analytical testing, world class lab capabilities, ongoing consultation, and more.
https://drugdeliverysystems.bd.com/contact-us
BD and the BD Logo are trademarks of Becton, Dickinson and Company © 2021 BD. All rights reserved.
-
Brochure BD PartnerPath™️ Program - Creating a clear pathway for the commercialization of your combination product
Learn about BD’s answer to the many challenges faced by pharmaceutical companies having new combination products to launch: our BD PartnerPath™️ program created for customers who value speed to market.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and PartnerPath are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Brochure BD capabilities to support pharmaceutical industry’s COVID-19 response
Discover how BD can accompany a development team working on drugs or vaccines against COVID-19, thanks to the specific expertise of its different businesses.
https://drugdeliverysystems.bd.com/contact-us
BD and the BD Logo are trademarks of Becton, Dickinson and Company © 2021 BD. All rights reserved. -
Brochure Administration challenges: Prefillable solutions in IV applications
Discover BD offering to address the challenges of IV drug delivery and find the right primary container for your injectable in hospital care settings: BD Hylok™️ Glass Prefillable Syringe for IV injected drugs or BD Sterifill Advance™️ Polymer Prefillable Syringe!
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo, Hylok and Sterifill Advance are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Brochure The Difference of One Drug Delivered - Portfolio Brochure
Learn about our portfolio of solutions and services for a wide variety of therapeutic areas and settings: from home to hospital through outpatient clinic, and from vaccines to chronic disease treatments through small molecules and specialties, dermal fillers and contrast media… We provide unparalleled parenteral drug delivery systems, including glass and plastic syringe systems, self-injection devices, safety and shielding systems that set the standard.
Discover why hundreds of pharmaceutical and biotechnology companies place their confidence in our high performing BD products and services...
https://drugdeliverysystems.bd.com/contact-us
BD and the BD Logo are trademarks of Becton, Dickinson and Company © 2021 BD. All rights reserved. -
Brochure Brochure BD Prefillable Syringe Solutions
Discover why hundreds of pharmaceutical and biotechnology companies place their confidence in our high performing BD products and services...
https://drugdeliverysystems.bd.com/contact-us
-
Brochure Brochure BD Service Offering
At BD, we have built our services and capabilities around a deep understanding of the drug development process and the key objectives at each stage. Knowledgeable and responsible, our team offers the many capabilities you need beyond taking your product to market. Each of our services is delivered intentionally and carefully to directly result in significant benefits for your product and company.
Discover why hundreds of pharmaceutical and biotechnology companies place their confidence in our high performing BD products and services...
https://drugdeliverysystems.bd.com/contact-us -
Brochure Brochure BD Technical Services
Discover our capabilities and services, and partner with us to:Save time by leveraging our tried and tested expertise and methods Save money by eliminating the need to invest in costly machinery and other resources Gain confidence by working with an organization that has successfully helped launch hundreds of drug products Free-up valuable internal resources that may be deployed to other critical activities.For more information, contact [email protected] or https://drugdeliverysystems.bd.com/contact-us
-
Brochure Brochure BD Hypak™ for Vaccines Glass Prefillable Syringe
Our prefillable vaccine syringes provide multiple routes of administration options while helping enhance vaccination outcomes.
BD Hypak™ for Vaccines Glass Prefillable Syringe is our reliable glass prefillable syringe system specifically optimized for vaccinations. BD Hypak™ for Vaccines addresses customer needs of better processability of your syringe during filling and packaging operations.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and Hypak are trademarks of Becton, Dickinson and Company © 2020 BD. All rights reserved. -
Brochure Brochure BD Hylok™ Glass Prefillable Syringe For Intravenous Drugs - Make it a part of your drug launch strategy Glass Prefillable Syringe for Intravenous Drugs
BD Hylok™ Glass Prefillable Syringe for IV Injection is a new luer syringe for the administration of IV drugs. It offers customers the advantages of glass containers (e. g., medication stability) with a robust luer connection. BD Hylok™ is a ready-to-use glass solution, compatible with main IV connectors. Discover how BD Hylok™ can support your prefilled syringe “First-Mover” strategy.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and Hypak are trademarks of Becton, Dickinson and Company © 2020 BD. All rights reserved. -
Brochure Brochure BD Neopak™ Glass Prefillable Syringe For Biotech - Turning expertise into advanced solutions for biotech
BD Neopak™ Glass Prefillable Syringe platform is built on years of BD’s unmatched experience partnering with leading biopharmaceutical companies to support drug development and launch. BD Neopak™ is conceived with a solution-finding mindset, allowing to tangibly meet your current and future needs for biotech drugs.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and BD Neopak are trademarks of Becton, Dickinson and Company © 2020 BD. All rights reserved.
-
Brochure Brochure BD Neopak™ Glass Prefillable Syringe For Biotech (Innovators)
Give tomorrow’s most demanding biotech drugs the strength of BD Neopak™ Prefillable Syringe technology!
BD Neopak™ Glass Prefillable Syringe platform is built on years of BD’s unmatched experience partnering with leading biopharmaceutical companies to support drug development and launch. BD Neopak™ is conceived with a solution-finding mindset, allowing to tangibly meet your current and future needs for biotech drugs.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and BD Neopak are trademarks of Becton, Dickinson and Company © 2020 BD. All rights reserved.
-
Brochure Brochure BD Neopak™ Glass Prefillable Syringe For Biotech (Life Cycle Management)
Change to the reliability of BD Neopak™ platform to accompany your brand strategy!
BD Neopak™ Glass Prefillable Syringe platform is built on years of BD’s unmatched experience partnering with leading biopharmaceutical companies to support drug development and launch. BD Neopak™ is conceived with a solution-finding mindset, allowing to tangibly meet your current and future needs for biotech drugs.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and BD Neopak are trademarks of Becton, Dickinson and Company © 2020 BD. All rights reserved -
Brochure Brochure BD Neopak™ Glass Prefillable Syringe For Biotech (Biosimilars)
When time to market is critical, invest in the reliability of BD Neopak™ Glass Prefillable Syringe For Biotech!
BD Neopak™ Glass Pre-fillable Syringe platform is built on years of BD’s unmatched experience partnering with leading biopharmaceutical companies to support drug development and launch. BD NeopakTM is conceived with a solution-finding mindset, allowing to tangibly meet your current and future needs for biotech drugs.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and BD Neopak are trademarks of Becton, Dickinson and Company © 2020 BD. All rights reserved. -
Brochure Brochure BD Neopak™ XSi™ Glass Prefillable Syringe - Helping you address silicone-related concerns
Concerned about silicone?
Discover BD Neopak™ XSi™ Glass Prefillable Syringe with immobilized Silicone Coating to help you address silicone-related concerns!
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo, BD Neopak and XSi are trademarks of Becton, Dickinson... -
Brochure Brochure BD Hypak™ for Biotech Glass Prefillable Syringe
Grounded on decades of BD Hypak™ experience, BD Hypak™ for Biotech has been developed to address the key compatibility challenges for biotech drugs and chronic treatments.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and Hypak are trademarks of Becton, Dickinson and Company © 2020 BD. All rights reserved. -
Brochure Brochure BD Vystra™ Disposable Pen - Expertise and efficiency combined
Discover BD Vystra™ Disposable Pen, an intuitive, high-quality and customizable pen injector designed to support therapies that require frequent, low-volume injections or variable dosing.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and BD Vystra are trademarks of Becton, Dickinson and Company © 2020 BD. All rights reserved.
-
Brochure Innovating in the viscosity design space - New 2.25 mL autoinjector platform
Extending its patient-centric BD Intevia™️ Disposable Autoinjector platform, BD builds on past successes while meeting the requirements of new, highly viscous, high-volume biologics for chronic diseases.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo, Intevia, Physioject, Neopak and XtraFlow are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Brochure The search for drug dose optimization (The Pharma’ Post n° 5 - Feb. 2021)
Interview of Marie-Liesse Le Corfec, Head of Global Portfolio Marketing at BD Medical - Pharmaceutical Systems, a leader in injectable drug delivery devices that is also in charge of the operational Task Force for coordinating the response and strategy with the pharmaceutical industry in the context of the health crisis.
https://drugdeliverysystems.bd.com/contact-us
BD and the BD Logo are trademarks of Becton, Dickinson and Company © 2021 BD. All rights reserved. -
Brochure The Difference of BD Solutions at Hospital, at Outpatient Clinic & at Home
BD provides unparalleled parenteral drug delivery systems for a variety of therapeutic areas and settings, including glass & plastic syringes, advanced drug delivery solutions, safety & shielding systems that set the standard. Discover why hundreds of pharmaceutical and biotech companies place their confidence in BD high performing products and services.
https://drugdeliverysystems.bd.com/contact-us
BD and the BD Logo are trademarks of Becton, Dickinson and Company © 2021 BD. All rights reserved. -
Brochure BD Medication Delivery Solutions: Brochure BD MDS for Industry Partners - Enabling preparation and administration of injectable drugs worldwide
From drug discovery, through manufacturing and quality controls, packaging and storage to preparation and administration, BD MDS delivers reliable and innovative solutions. Discover our offering for the industry!
Contact: BD Medication Delivery Solutions (Europe) - Route De Crassier, 17 - 1262 Eysins, Vaud - Switzerland - T: +41 21 556 30 00
BD, the BD Logo and all other trademarks are property of Becton, Dickinson and Company © 2020 BD. All rights reserved
-
Brochure BD Medication Delivery Solutions: Brochure BD Eclipse™ - Designed and manufactured with nurses and patients in mind
Discover BD Eclipse™, designed and manufactured with nurses and patients in mind! BD Eclipse™ needle is easy to use with minimal training, and supports streamlined and cost effective compliance for every practitioner, every day. BD Eclipse™ family of needles is a proven technology as it meets testing requirements for internationally recognized ISO 23908 standard for sharps injury protection with hypodermic injection needles.
Contact: BD Medication Delivery Solutions (Europe) - Route De Crassier, 17 - 1262 Eysins, Vaud - Switzerland - T: +41 21 556 30 00
BD, the BD Logo and BD Eclipse are trademarks of Becton, Dickinson and Company © 2020 BD. All rights reserved -
Brochure BD Medication Delivery Solutions: Brochure BD Injection Expertise - Pioneer and expert in the development of safety-engineered medical devices
BD has developed an extensive line of safety-engineered devices, becoming a global leader in the field of healthcare workers safety. Discover our innovative portfolio of safety products protecting against needle stick injuries.
Contact: BD Medication Delivery Solutions (Europe) - Route De Crassier, 17 - 1262 Eysins, Vaud - Switzerland - T: +41 21 556 30 00
BD, the BD Logo and all other trademarks are property of Becton, Dickinson and Company © 2020 BD. All rights reserved
-
Technical Data BD investing $1.2 billion into manufacturing of prefillable injection systems
BD has plans to invest approximately $1.2 billion over a 4-year period. The investment will fund capacity expansion, new product innovations, manufacturing technology enhancements and business continuity improvements across its existing network, all designed to maximize supply and reduce risks for pharmaceutical companies that rely on ready-to-fill syringes for their injectable drugs - including complex biologics, vaccines and small molecules -. A new manufacturing facility in Europe is expected to be operational by the end of 2023.
More information available in our datasheet.
https://drugdeliverysystems.bd.com/contact-us
BD and the BD Logo are trademarks of Becton, Dickinson and Company © 2021 BD. All rights reserved.
-
Whitepaper Marie-Liesse Le Corfec's interview by the Pharmaceutical Post (October 2021)
"The crisis was a pilot phase during which we learned more about the strength of our systems"
Interview given by Marie-Liesse Le Corfec, Marketing Director of the BD Medical - Pharmaceutical Systems' portfolio, who leads an internal Task Force in order to coordinate the response and strategy targeting the pharmaceutical industry during the health crisis -
Whitepaper Investigating Performance of Glass Prefillable Syringes (PFS) in Deep Cold Storage
While traditional vaccine formulations are commonly stored up to about 2°C-8°C, new vaccine formulations – including those for mRNA – require much colder storage for over a long period of time to ensure drug-product shelf-life and potency. Such low temperatures can induce changes in container dimensions, phase transition - liquid to solid and vice versa - and thermal shock. This could compromise the functional performance of the delivery system and Container Closure Integrity (CCI). BD began its investigation on the impact of deep cold storage on glass PFS as soon as information regarding the deep cold storage requirement for mRNA COVID-19 vaccines became available in 2020.
BD study reveals that glass PFS can be stored at -20°C and -40°C without risk on PFS key product functions, establishing pathway to further assess the stability and performance of drug combination products with PFS in deep cold conditions.
Discover more information in our press release...
-
Whitepaper Selecting Drug Delivery Systems for Higher Doses, Higher Viscosities & Lower Risk
The development of new parenteral biologics with high-volume, high-viscosity formulations (> 1 mL, >15 cP) is triggering the need for devices that can deliver these therapies with the ease, safety, and low injection time required for self-injection. As these formulations come to market, pharmaceutical and biotech companies must make critical device choices from an array of largely unproven options. The attachment discusses how companies can de-risk their device selection as they bring this new generation of high-volume, high-viscosity biologics to market.
Discover the article published in Drug Development & Delivery in October 2021 (Vol 21 No 7)... -
Whitepaper The Future of Container-Level Traceability For Prefillable Syringes
In this article, Sam Eubanks, Vice-President of Software Development, and Hervé Soukiassian, Associate Director, R&D Project Management, both of BD Medical - Pharmaceutical Systems, discuss the BD traceability solution. This concept under development aims to deliver traceability at the unique container level from manufacture to the point of care.
https://drugdeliverysystems.bd.com/contact-us
BD and the BD Logo are trademarks of Becton, Dickinson and Company © 2021 BD. All rights reserved. -
Whitepaper BD Libertas™ effectively delivers dose volumes up to 5 mL subcutaneously
New clinical trial data demonstrates the performance of BD Libertas™ Wearable Injector. A detailed analysis of the BD independently sponsored and conducted study has been published in Clinical and Translational Science (5 Dec 2020).
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and Libertas are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved.
-
Whitepaper BD Hylok™ Glass Prefillable Syringe for IV applications
Sophie Trémeau, Regulatory Affairs Specialist, Maxime Nicolas, R&D Engineer, and Myriam Leszczynski, R&D Project Manager, all of BD Medical – Pharmaceutical Systems, discuss BD Hylok™, a prefillable glass syringe for intravenous applications, including the rigorous testing BD Hylok™ has undergone and the regulatory support BD is able to provide to its pharmaceutical partners.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and Hylok are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved.
-
Whitepaper Addressing the evolving needs of variable drug delivery regimens
Temitope Sodunke, PhD, Strategic Innovation Leader (On Drug Delivery - February 2020)
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and Evolve are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved.
-
Whitepaper BD completes clinical trial for BD Libertas™ Wearable Injector
Press release - February 11, 2020
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and Libertas are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved.
-
Whitepaper The changing drug delivery paradigm
Beth DiLauri, Director, Strategic Marketing, Advanced Drug Delivery Solutions (On Drug Delivery - September 2019)
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo, Neopak and Libertas are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Whitepaper System integration
Alice Maden, Associate Director, Regulatory Affairs & Lionel Maritan, Associate Director, Research & Development (On Drug Delivery - February 2019)
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo, Physioject, Intevia, Neopak and Libertas are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Whitepaper How shorter needles with thinner walls are set to improve the injection experience in chronic care
Aurélie Pager, Clinical & Human Factors Program Leader - Brigitte Duinat, Senior Engineer - Barbara Alves, Regulatory Affairs Specialist (On Drug Delivery - May 2020)
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo, Neopak and XtraFlow are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Whitepaper 8 mm needle - Improving subcutaneous chronic drug delivery
Aurelie Pager, Clinical & Human Factors Program Leader (On Drug Delivery - October 2019)
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo, Neopak, Intevia and Physiolis are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Whitepaper Hyaluronic acid dermal filler delivery in cosmetic applications
Sagar Bejalwar, Global Marketing Manager (On Drug Delivery - February 2019)
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and Hylok are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Whitepaper Validation studies of BD Sterifill Advance™ Polymer Prefillable Syringe
Cécile Halté, Senior Program Manager, Acute Care Segment (On Drug Delivery - August 2019)
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and Sterifill Advance are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Video BD Pharmaceutical Services & Solutions
Discover a comprehensive set of services and solutions designed to mitigate the risks associated with combination product development to help you achieve your goals. These services and solutions help you from development all the way through to launch, including container selection, combination product expertise and testing, regulatory and technical documentation support, analytical testing, world class lab capabilities, ongoing consultation, and more. -
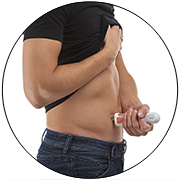
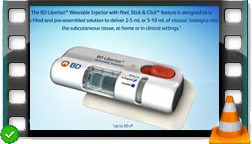
Video BD Libertas™ Wearable Injector - Introductory overview
BD Libertas™ Wearable Injector with Peel, Stick & Click™ feature is designed as a prefilled and preassembled solution to deliver 2 – 5 mL or 5 – 10 mL of viscous biologics into the subcutaneous tissue, at home or in clinical settings. Discover its main features and benefits!
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and BD Libertas are trademarks of Becton, Dickinson and Company or its affiliates © 2021 BD. All rights reserved. -
Video BD Vystra™ Disposable Pen - Manufacturing
BD Vystra™ Disposable Pen is an easy-to-use disposable pen injector for frequent, low-volume injections or variable dosing. The video details BD Vystra™ features and showcases the robust manufacturing process from component assembly through to final packaging for shipment.
https://drugdeliverysystems.bd.com/contact-us
BD, the BD Logo and BD Vystra are trademarks of Becton, Dickinson and Company © 2021 BD. All rights reserved. -
Video Trends in Pharma Packaging, Administration & Drug Delivery Devices
Faster, more cost-efficient hospital-based injectable drug delivery - How speed and convenience imperatives in the Covid-19 crisis have challenged the paradigms Overview and a discussion on the evolution of next-generation products Moderator: Yasemin Karanis, Consultant, European Thought Leadership, IQVIA Global Pharma: Trends in Drug Delivery and Formulations -Yasemin Karanis, Consultant, European Thought Leadership, IQVIA The increasing importance of connected devices and digitalization in post-pandemic R&D - Sai Shankar, Vice President, Global Digital Healthcare Systems, Aptar Pharma Illustration of implementing user experience key aspects and outlining holistic integrated combination products support - Mark Tunkel, Director of Business Development, Insight Innovation Center, Nemera Faster, more cost-efficient hospital-based injectable drug delivery - How speed and convenience imperatives in the Covid-19 crisis have challenged the paradigms - Sagar Bejalwar, Global Acute and Specialty Portfolio Leader, BD Medical – Pharmaceutical Systems Trends in sustainable packaging in the FMCG market - how this influences the Pharma business and ongoing developments - Armin Ullmann, Product Manager Sustainable Healthcare Laminates, Huhtamaki Flexible Packaging Sustainable packaging in Merck supply chain – ongoing and future developments - Corinne Ondo, Head of Sourcing Glass & Healthcare Innovation Group Procurement, Merck Healthcare This presentation was originally aired as part of the CPHI Festival of Pharma. -
Video Vaccine Development Challenges: Speeding Up and Scaling Up
The COVID-19 pandemic has lead governments, companies and research institutions to accelerate vaccine development efforts amid geopolitical crosscurrents. Despite an increased understanding of immune responses to infection, research is often hindered by a lack of knowledge of the immune responses explicitly required for protection, or by a lack of approved adjuvants and delivery systems to induce the needed answers. Licensing new vaccines also require a significant financial commitment which also represents a challenge. This webinar will discuss the hurdles that new vaccines must overcome to reduce morbidity and mortality, as well as some of the initiatives that are being attempted to accelerate and scale up the next generation of vaccine development for global disease prevention. This presentation was originally aired as part of the CPHI Festival of Pharma. -
Video Vial to Ready-To-Administer Need and BD Solution Offerings in Acute Care
This webinar originally aired as part of CPHI Discover - 17-28 May 2021 Several factors motivate pharmaceutical companies and hospitals to use glass pre-fillable syringes (PFS) for Intravenous (IV) applications. By switching from vials and ampules to a glass PFS, hospitals have the potential to reduce medical errors, improve workflow efficiency and impact ROI for hospital stakeholders. Moreover, data suggests that approximately 92% of hospital stakeholders are willing to use prefilled syringes for multiple drugs. For pharmaceutical companies, transitioning a fixed-dose drug from its vial or ampule format to a prefilled syringe can serve as a differentiation strategy in a market dominated by vials and ampules. This session will examine the benefits and challenges associated with glass formats and will explore BD’s glass pre-fillable syringe with connection performance verified for IV applications. -
Video Patient-Centric Drug Delivery Solutions for Self-Administration
This webinar originally aired as part of CPHI Discover - 17-28 May 2021 The need to reduce health care costs, improve outcomes, and increase access to remote care are intensifying the focus on patient care delivery in the home setting. As a result, pharma is seeking alternate delivery systems that can provide patient autonomy and mobility while delivering treatment outside of the clinic. Programmable, variable dose on-body injectors provide such options. This talk will highlight considerations for on-body injectors to promote drug delivery in non-clinical settings. -
Video COVID-19: Delivering Vaccines to the Masses
This webinar originally aired as part of CPHI Discover - 17-28 May 2021 While the initial challenge was in developing effective therapies against COVID-19, the next hurdles are manufacturing at scale, drug delivery solutions, cool chain and logistics. How is the industry responding? COVID Update: The 2021 Vaccine Line Up Facility challenges & production quality control Cold chain challenges Administration solutions-what are the options vs standard injections? Key supply chain challenges for vaccine products Creating sustainable and resilient distribution systems. Challenges in allocating and prioritizing vaccine supplies and distribution -
Video Interrogating Large Volume Subcutaneous Injection Using Translational Preclinical and Clinical Models and the Case Study “Clinical Evaluation of an Investigational 5mL Wearable Injector
Learnings from preclinical and clinical translational studies using fluoroscopic (preclinical) or ultrasound (clinical) in situ imaging to characterize 0.6-10 mL, 1-50 cP subcutaneous injections delivered by a surrogate pump or an investigational on-body injector Session Outline: Assess the feasibility and/or the tolerability (clinical only) of SC injections up to 10 mL & 50 cP Provide detailed in situ device and depot imagery to reveal what happens beneath the skin surface during drug delivery Explore the relationship between visible tissue effects and underlying depots Qualify and quantify the impact of ambulatory wear on wearable and on-body injector performance and the corresponding tissue effects and subject perception (wearable injector only) -
Video Addressing the Evolving Needs of Complex or Variable Drug Delivery Regimens
The need to reduce health care costs, improve outcomes, and increase access to remote care are intensifying the focus on patient care delivery in the home setting. As a result, pharma is seeking alternate delivery systems that can provide patient autonomy and mobility while delivering treatment outside of the clinic. Programmable, variable dose on-body injectors provide such options. This talk will highlight considerations for on-body injectors to promote drug delivery in non-clinical settings. This webinar originally aired as part of Pharmapack 2021 -
Video Combination Product Development Considerations for Subcutaneous 2 mL Dose Delivery
High dose biologic formulations are pushing drug volume and viscosity beyond traditional self-injection design space limits (> 15 cP, > 1 ml dose volume), which can challenge patients’ capabilities to administer their injections based on injection time or force. We will discuss how ergonomic, manual, syringe-based injections can enable subcutaneous self-injections with characteristics that go beyond the traditional boundaries of volume and viscosity. Important factors for the development of combination products involving delivery of higher volumes (1-2 mL) are presented, including assembly, human factors, documentation, and platform considerations.This webinar originally aired as part of Pharmapack 2021 -
Video Unit Level Syringe Identification & Traceability
BD has teamed up with industry-leading partners in tagging and software technologies to develop a comprehensive solution to deliver traceability at the unique container level. The immediate focus is on pharma manufacturing operations with the goal being to ultimately ensure the integrity of the drug product throughout the supply chain and at the point of consumption. BD would like to assist pharma customers in transitioning their manufacturing lines to unit-level traceability with a coherent path towards end-to-end traceability. This presentation will discuss how interested customers are invited to specify the use case requirements to be built into the traceability solution and test the tagged syringes, production line hardware and software in a pilot project.This webinar originally aired as part of Pharmapack Europe 2021 -
Video Responding to the Vaccine Delivery Challenge
The devastating global impact of the present pandemic has revealed the challenge of vaccine delivery, and the critical role of packaging and administration material. The 30-minute talk will focus on how prefilled syringes contribute to solve challenges of the vaccine administration. This will include a review of the efficiency gains and waste reduction obtained with the PFS format, with benefits in terms of the workflow efficiency, dose-sparing and waste volume reduction. Finally, drug/container compatibility issues will be explored along with the suitability of PFS technology in meeting the rigours of vaccine cold/frozen chains storage. This webinar originally aired as part of Pharmapack 2021 -
Video CPHI WW: BD Services & Solutions - Develop. De-risk. Deliver.
This session introduces a comprehensive set of services and solutions designed to mitigate the risks associated with combination product development to help you achieve your goals. These services and solutions help you from development all the way through to launch, including container selection, combination product expertise and testing, regulatory and technical documentation support, analytical testing, world-class lab capabilities, ongoing consultation, and more. -
Webinar Proof of Processability for New Coated Stopper on Filling and Assembly Lines. A Collaborative Study by Bausch+Ströbel and BD Medical – Pharmaceutical Systems
In this webinar, originally broadcast as a part of Pharmapack Europe, Eve-Marie Vaccaro, Global Sales & Operation Planner, and Edgar Bauer, Global Key Account Manager Bausch+Ströbel from BD Medical - Pharmaceutical Systems discuss how VHP sterilization is a viable alternative for ensuring improved patient safety by providing a sterile product application for drug delivery devices, such as pre-filled syringes with temperature or radiation sensitive drug products, that are too sensitive for conventional methods of sterilization. Material selections, device design, properties of the pre-filled syringe, and secondary packaging decisions (shape, materials) play an important role in ensuring the sterilization process is successful. The major things to avoid are known in the industry, but there are more opportunities for proactively preparing for this in advance, during the design phase, when making considerations for the intended sterilization method - for example - thinking about applying the IPCD (internal process challenge device and EPCD (external process challenge device) in good time beforehand. These are key considerations when aiming at short lead-time product-to-market strategy - from sterilization perspective. This presentation looks at these from this viewpoint. -
Video Essai
kdsjqkdjgdkmsjgksmdlgjlkmjdqgkm -
Webinar Panel Discussion - The Future of Large-Volume Drug Delivery
This session will bring together industry experts to discuss the opportunities and challenges that large-volume delivery devices have moving forward. Balancing technical and regulatory demands with ever-increasing pressures on cost-effective and sustainable solutions needs continued consideration. These topics will be discussed along with how to meet patient and healthcare expectations. -
Webinar Keynote Address
Where are all the On Body Delivery Devices? On Body Large Volume Injectors (LVI) have been discussed and demonstrated for over ten years, but very few of these devices have reached the hands of patients. Two of CDPs combination product experts will outline the barriers that these products have faced in coming to market and the competition that large volume auto-injectors and ambulatory infusion pumps present. The presentation will include commentary on the relevant drug product pipelines, the regulatory pathway, relevant standards and future drivers for successful LVI development. -
Webinar Lightning Talk - Ecosystem Considerations in Adopting Large-Volume Onbody Injectors
As the landscape, development, and introduction of large-volume delivery systems continue to grow, the implementation of these devices in the Pharma ecosystem creates unique challenges for pharmaceutical companies. From the selection of drug containment materials and enclosures, to device assembly needs to final packaging, the ecosystem and implementation of these devices must be carefully considered. In this quick discussion we will review some of the ecosystem considerations below. Evolving landscape vs uniformity Device complexity and options, User Filled, User Assembled, Prefill-Preassembled Drug containment choices and second source de-risking Post fill device assembly, what complexity does this introduce and do the device companies provide turn key solutions. Device packaging and distribution options. Lifecyle management especially related to electronics/connected systems Sustainability opportunities and disposability -
Webinar Lightning Talk - From Hospital to Home: Adapting to a New Era of Healthcare
The healthcare landscape is experiencing a significant shift as traditional hospital-based care transitions to the home where feasible, enabled by innovative technologies supporting remote care, monitoring, and patient engagement. This talk aims to underscore the importance of preparing for this evolving era of healthcare, and how to ensure new medical device designs are suitable for use at home. Key discussion points include: Current adoption levels, case studies, and future trends surrounding "Hospital at Home" or "Virtual Wards" in the UK/EU. Key considerations and challenges in developing or adapting technology for home-based care, such as user-friendly interfaces and interoperability. The potential impact of technology designed for home use on patient outcomes, cost savings, and overall experience, exemplified by a case study on new infusion pumps. -
Webinar Lightning Talk - The Challenges of Scaling Novel Sustainable Cold Chain Packaging Solutions
Product/patient safety is paramount, so how to balance the selection of packaging materials and systems with sustainability in mind? More sustainable solutions are coming online in different forms and need to be scaled into the market. Challenges on the innovation side focus on the validation of important to have the integration between validation and monitoring to give comfort to industry to embrace flexibility and sustainability. What low impact solutions can work for the pharma industry. In an ideal world, a one size fits all would be found, but the reality is that shipping lane profiles and product requirements means that this is unlikely to be the case. -
Webinar Transforming Pharma Fill/Finish Processes with RFID - Enabled Pre-fillable Syringes (PFS): Use Cases and Live Demo
Sterile pharmaceutical production is expected to grow by over 50% in the next seven years, driven by new demand for recombinant antibodies and small molecules. Consequently, the fill/finish stage in pharmaceutical manufacturing is becoming a bottleneck in the pharmaceutical production . Health authorities and regulators are also demanding enhanced traceability during the manufacture of parenteral medicines as the most common processes and technologies in place are reaching their limitations . Addressing this challenge means boosting Overall Equipment Efficiency (OEE) while meeting rising regulatory demands. This presentation explores an innovative solution to address this dual challenge: Container-level traceability (CUID). This pioneering approach utilizes RFID (Radio Frequency Identification) to ensure accountability for each container in the fill-finish processes, down to the individual unit. In the short term, CUID can mitigate exposure to mix-up, improve OEE through automated reconciliation, and cut waste with unit-by-unit segregation. Beyond this, CUID has the potential to eliminate data silos and bridge the gap between each manufacturing process step. Manufacturers can harness advanced data analytics to unveil hidden patterns, identify process inefficiencies, and detect trends and anomalies that were previously unnoticed. Becton Dickinson selected RFID technology for pre-fillable syringes based on the relatively low impact on existing manufacturing lines and the high potential for future applications. In collaboration with Avery Dennison Smartrac, a leading provider of RFID inlay solutions, BD has conducted a proof of concept and will demonstrate the feasibility of the solution through a video of BD’s RFID Demonstration Lab and a live demo. -
Webinar Oxygen Permeability Considerations and Performance for Prefillable Syringes
Numerous drug technologies are advancing into pre-clinical and early clinical phases to enable new therapies with improved health outcomes. Those new technologies carry potential challenges pertaining to their compatibility with the container materials and with environmental conditions such as temperature or exposure to gases. One historically common degradation pathway of pharmaceuticals is oxidation. More and more prefillable plastic syringes are being developed as they present advantages over glass syringes on parameters such as lower inorganic extractables and a higher resistance to breakage. Plastic syringes are mostly made of COP and COC barrel materials, materials which are much more permeable to oxygen than glass. A highly oxygen-permeable container results in a faster oxygen ingress, leading to faster oxidation. For oxygen-sensitive drug formulations, this can lead to a decrease in the drug shelf life. Decreased shelf life may be mitigated by increasing the concentration of antioxidants or by adding an oxygen scavenger in the secondary packaging, which can pose other constraints. Therefore, there is a need to monitor and quantify the oxygen ingress within prefillable syringes to derisk pharma combination product development. This study, conducted on several prefillable syringe configurations, will highlight the influence of components on oxygen permeation and measure the syringe protection performance against oxygen ingress at different storage temperatures. -
Webinar Precision Drug Delivery: Innovation in High-Dose, Large-Volume Applications
Today’s medicine and therapeutics encompass fairly complex formulations. The device design needs to not only incorporate the challenging needs of the formulation but also address patient optimized administration and deliver consistent results. The success of the clinical outcome is dependent on the delivery optimization in these situations and thus the importance of methodologies to be able to monitor delivery and evaluate Pharmacokinetics (PK) modeling. The presentation will highlight novel methodologies developed to explore drug characterization, drug delivery optimization, and autoinjector simulator to assess pharmacokinetics / pharmacodynamics towards early evaluation and consistent results. The methodology can be used for novel therapies and unmet needs in various indications including the areas of high dose, large volume and high viscosity applications. Some case studies will be highlighted with the methodology. Topics include aspects of: Development process for Drug Delivery Delivery Evaluation – Drug, Site, PK-PD modeling Path to Device – Injection Parameters Optimization Integrated Development Cycle -
Webinar De-risking combination product development for large volume biologics: a pharma partner’s perspective
Transitioning drugs from IV to subcutaneous (SC) delivery brings new requirements and impacts program considerations for combination product development. In this session, we identify trade-offs that pharma weighs to de-risk combination product development programs involving large volume subcutaneous (LVSC) delivery. Key issues can include: Understanding the patient experience throughout the injection process with large volume subcutaneous delivery Reducing risk of user variability to gain market confidence in successfully delivering LVSC formulations Leveraging partner capabilities, insights, and data to inform combination product trade-offs and program risks Manufacturability of the combination product including the delivery system and integration into the pharmaceutical operations network Exploring the value of drug delivery technology used consistently from clinical development into commercial use Examples of how these issues can impact a combination product development program may be explored. -
Webinar Enabling Digital Health Solutions with the Connected BD UltraSafe Plus™ Passive Needle Guard
Sub-optimal adherence and persistence to treatment continue to be significant challenges for both on-market drugs and investigational products in clinical development. This is especially true for self-administered medications for chronic disease patients, hence driving the need for improved treatment intake monitoring technologies. To answer this need, BD and Biocorp have joined forces to develop a connected version of the BD UltraSafe Plus™ Passive Needle Guard that facilitates the traceability of injections. During this workshop, the speakers will welcome you for an interactive presentation featuring the following topics: • Unmet needs in clinical & real-life settings: the challenge of treatment adherence • The solution: overview of the connected BD UltraSafe Plus™ Passive Needle Guard, technical details and value proposition for different stakeholders • Interactive demo session • Use cases: how the connected BD UltraSafe Plus™ Passive Needle Guard can fit into clinical and real-life practices -
Webinar Injection Time and Usability Considerations for Self-Injection Systems, a BD Perspective
As the number of patients managing chronic diseases continues to increase, self-injection solutions play an important role in enabling the shift towards home care. Identification of potential use errors is a critical task during device development to support patient acceptance and usability. The design of these solutions can help overcome some of the usability challenges associated with self-injection, such as injection time limits and risk of premature device removal. As biologic dose volume and viscosity increases, meeting injection time requirements and usability considerations with autoinjectors can be increasingly challenging. During this session, we will discuss device design elements that can impact the hold time of autoinjectors and the resulting usability considerations, such as the gap between second click and actual end of dose. Premature device removal is one example of usability errors that can result from the second click occurring before actual end of dose. Design alternatives to avoid risk of premature device removal during self-administration will be explored including that of the BD Physioject™ Disposable Autoinjector and the BD Libertas™ Wearable Injector. -
Webinar Introduction to Human Factors Engineering
Human Factors Engineering (HFE) process participates in the product development for a safe and effective use by the end-user population. HFE data are under agencies' scrutiny as part of the design control of a drug - device combination product. Timely planning can be challenging during combination product development. Session outline: HFE definition and overview of the regulatory landscape Presentation of formative and summative studies process Benefits and planning of HFE activities during combination product development Benefit: Beyond the definition of Human Factors Engineering, understanding how studies related activities impact the final product design and how to plan for timely data generation during combination product development. -
Webinar Clinical evaluation of an investigational 5mL wearable injector
An investigational large volume subcutaneous wearable injector delivered 5mL viscous placebo injections to healthy adults with favorable subject tolerability and acceptability. This session outlines:
An investigational large volume subcutaneous wearable injector was evaluated for feasibility of 5mL, ~8cP non-Newtonian placebo injections in abdomen and thigh of 52 healthy adults of two age groups (18-64, 65+ years), with and without movement. Assessment of device performance, tissue effects, depot location and user-reported pain and acceptability.
Benefit: Greater understanding of clinical performance and user perception of this investigational wearable injector may de-risk clinical outcomes as more biotherapies for chronic disease transition from intravenous to subcutaneous administration -
Webinar The next generation vaccine syringe - BD Effivax™ Glass Prefillable Syringe
As the vaccine market continues to grow, vaccine manufacturers are looking to differentiate themselves by seeking solutions for critical challenges that:
Offer end to end vaccine container reliability. Consider cost sensitivity. Support documentation for regulatory scrutiny. Ensure continuity of supply.
BD Effivax™ Glass Prefillable Syringe has been designed in collaboration with leading pharmaceutical companies to meet critical needs such as product integrity, cosmetic quality, and product consistency. It sets new standards of performance that improves end-to-end syringe reliability and can translate into lower total cost of ownership.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance











































































































![BD Services & Solutions - Develop. De-risk. Deliver. [Brochure]](https://www.cphi-online.com/Thumbnail%20BD-32696%20Services%20Intro%20Interactive%20Brochure-file120823.jpg)







































-file122588.png)
-file122589.png)
-file122590.png)
-file122534.png)
-file122587.png)



-file145522.png)
-file145515.png)
-file145518.png)
%20(1)-file145519.png)
-file145520.png)
-file145521.png)

-file145543.png)
%20(1)-file145612.png)
-file145663.png)





