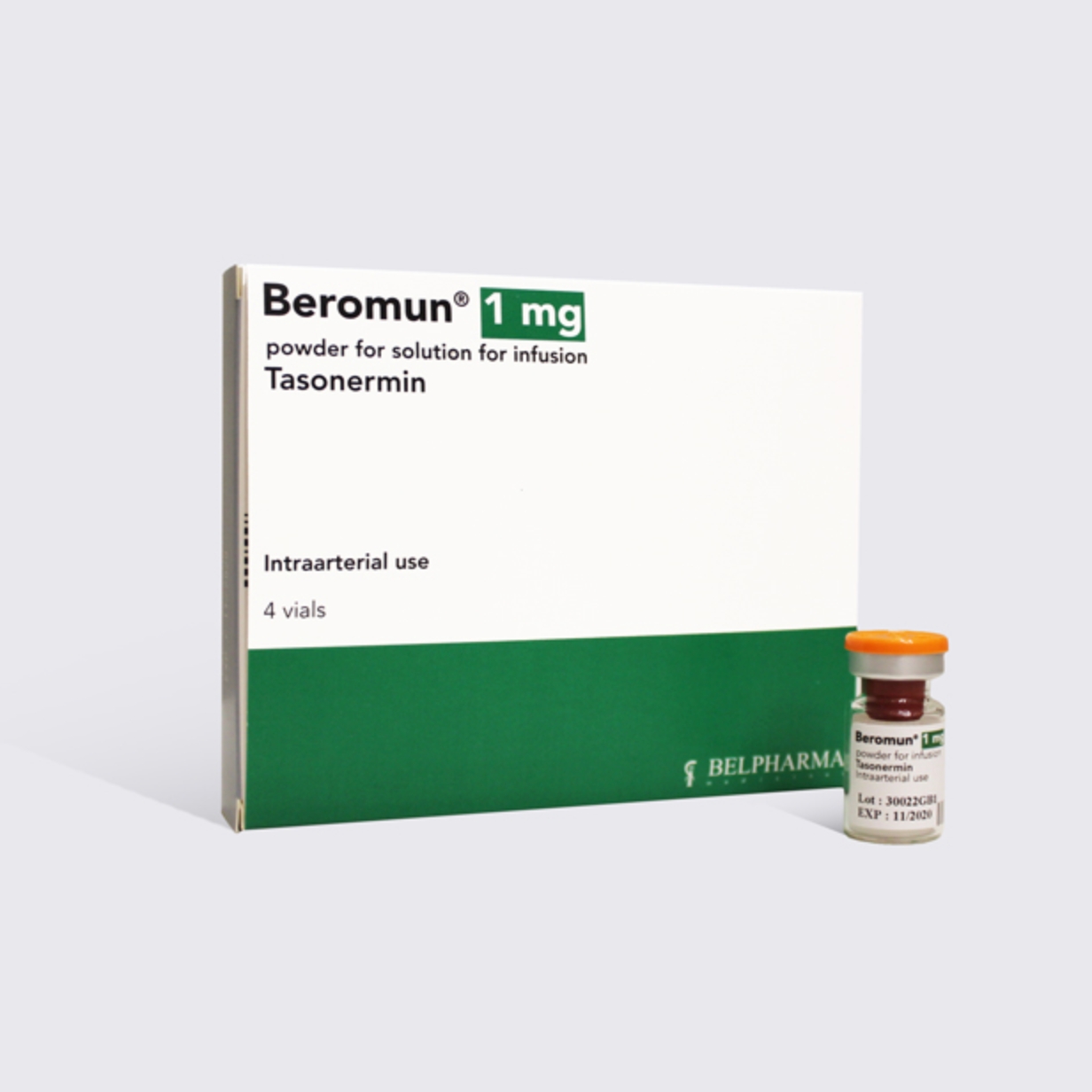
Beromun® is brand name for recombinant TNF-alpha – TASONERMIN (Produced by genetically modified E. Coli)
Beromun® is indicated in adults as an adjunct to surgery for subsequent removal of the tumor to prevent or delay amputation, or in the palliative situation, for non resectable STS of the limbs, used in combination with Melphalan via mild hyperthermic Isolated Limb Perfusion (ILP).
Beromun® is commercialized by BELPHARMA since December 2017, and is registered in EMA, Switzerland and Mexico.
Beromun® is also available worldwide on a case by case basis (such as named patient programs) via our distribution network.
Due to its very specific procedure of administration, Beromun® usage is restricted to experts centres in the treatment of Sarcoma with demonstrated expertise in performing ILPs.