- Home
- Biopharmaceuticals
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products551,744
-
Companies7,781
-
Articles11,636
-
Events8
-
Webinars342
Biopharmaceuticals
- Academia and Research Centres
- Analytical Chemistry
- Analytical Methods
- Antibody Drug Conjugates (ADCs)
- Antisense, RNA
- Assays
- Bio Clusters
- Bio process technologies
- Bioelectronics
- Bioinformatics
- Biomanufacturing
- Biopharmaceuticals (general category)
- Bioreactors & Fermentation
- Biosimilars
- Biotechnology and related specialities
- Capacity challenges
- Cell & Gene therapy
- CHO and cell lines
- CMC
- Continuous Bioprocessing
- Critical quality attributes
- Diagnostics
- Downstream
- Expression platforms
- Genomics & Proteomics
- Immunology
- Leachables/Extractables/Particulates
- Medicinal Chemistry
- Molecular Biology
- Monoclonal Antibodies
- Platform technologies
- Process validation
- Protein chemistry
- Regenerative Medicine
- Tech transfer and Scale up
- Technology and software
- Tissue Culture Medias
- Toxicology / Virology
- Upstream
- Viral Clearance
Biopharmaceuticals Companies (164)
Biopharmaceuticals News
-
News Women in Pharma: Diversi‘tea’ at CPHI North America
CPHI North America will unite the pharmaceutical supply chain in Philadelphia from May 7–9, 2024 for 3 days of innovation and connections. As part of the content Agenda, our Diversity Track will bring the industry together to discuss the imperati...23 Apr 2024 -
News Western pharma groups warn of supply disruptions over China anti-spy law
China’s anti-espionage laws have caused concern among western pharmaceutical groups over potential arrests or denial of access for foreign inspectors in China-based facilities and manufacturing partners, posing a risk to the supply of drug produc...22 Apr 2024 -
News CPHI Online Webinar Series – Optimising Pharma Manufacturing through Digital Transformations
This month’s CPHI Webinar Series explored achieving manufacturing excellence in pharma through the digitalisation of daily processes. Presented by Joe Doyle, Head of Sales at EviView, and Bikash Chatterjee, President and Chief Scientific Off...22 Apr 2024 -
News More to celebrate at the CPHI Milan Pharma Awards 2024
The CPHI Milan Pharma Awards, which are now open for nominations until 18 May 2024, celebrate the innovators making a difference. This year, two new categories have been added to further honour the contributions of a diverse range of pharma professiona...10 Apr 2024 -
News First offers for pharma from Medicare drug price negotiations
Ten high-cost drugs from various pharma manufacturers are in pricing negotiations in a first-ever for the US Medicare program. President Biden’s administration stated they have responded to the first round of offers.5 Apr 2024 -
News Coronary drug-coated medical device receives US FDA approval
Boston Scientific announced the FDA approval of their medical device AGENT Drug-Coated Balloon (DCB) for the indication of coronary in-stent restenosis in coronary artery disease patients.5 Mar 2024 -
News CPHI Online Webinar Series – Innovative Strategies for B2B Pharma Marketeers
On February 20, 2024, CPHI Online hosted a webinar on Innovative Strategies for B2B Pharma Marketeers. Featuring expert speakers from across the pharmaceutical value chain, this webinar delves into how B2B pharma marketeers can create better content to...28 Feb 2024 -
News 5 African American Pharmaceutical Pioneers: Honouring Black History Month
In recognition of February as Black History Month in the United States, we cover the lives and works of five pioneering African American pharmaceutical professionals who have contributed significantly to the world of pharmaceuticals and drug devel...21 Feb 2024 -
News Special Edition Women in Pharma: International Day of Women and Girls in Science
In this monthly series, we bring insightful content, events, and communities dedicated to sharing best practices for DE&I strategies and showcasing underrepresented voices in the pharmaceutical community.7 Feb 2024 -
News Novo Holdings buys CDMO Catalent in US$16.5 billion deal
Novo Nordisk’s holding and investment parent company will acquire the publicly owned CDMO at the end of 2024, taking it private and selling three of its drug manufacturing facilities to Novo Nordisk.7 Feb 2024 -
News Pharmapack Awards 2024 Start-up Innovation Award Winner – Capa Valve Ltd
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024.2 Feb 2024 -
News Pharmapack Awards 2024 Patient-Centric Design Award Winner – Dr Ferrer BioPharma
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024.30 Jan 2024 -
News Women in Pharma: Minding the Gap at Pharmapack 2024
2024 marks the first year Pharmapack will host a Diversity track dedicated to bridging the gap within the pharmaceutical packaging and drug delivery sector. The track includes a panel discussion on 'Enabling Diversity in the Workplace,' focused...29 Jan 2024 -
News Pharmapack Awards 2024 - Celebrating Packaging and Drug Delivery Innovation
The 2024 Pharmapack Innovation Awards ceremony celebrated the best in pharmaceutical packaging and drug delivery innovation at all levels. The awards were held on January 24, 2024 at the Paris Expo Porte de Versailles.24 Jan 2024 -
News On Track at Pharmapack 2024 - The Track Sponsor interview: BD Pharmaceuticals
January 2024 brings both a new year and Europe’s leading packaging and drug delivery event. Bringing the world’s experts in pharmaceutical packaging together in Paris, France, Pharmapack 2024 brings exciting opportunities to learn and colla...17 Jan 2024 -
News 2024 Pharma Industry Trends Outlook: Collaboration, Market Maturity, and Digital Futures
The annual CPHI Online 2024 Pharma Trends Outlook, in partnership with Arvato Systems, identifies 12 key industry trends shaping the life sciences industry in the coming year.9 Jan 2024 -
News New Novo Nordisk AI hub for drug discovery to open in London, UK
Danish pharmaceutical giant Novo Nordisk will be opening an AI-based research facility in the heart of London to advance drug discovery operations.5 Jan 2024 -
News BioNTech to begin mRNA vaccine manufacturing in Rwanda by 2025
German biotechnology company BioNTech has stated their intentions to begin production at their mRNA vaccine factory in Rwanda by 2025, which will mark the first foreign mRNA vaccine manufacturing site on the continent of Africa.20 Dec 2023 -
News Women in Pharma: Looking back on 2023 and moving forward to 2024
In this monthly series, we interview women from across the pharmaceutical industry and supply chain to discuss the importance of gender diversity in healthcare, the workplace, and beyond.19 Dec 2023 -
News CPHI Barcelona 2023: Partnering for Success – Managing Outsourcing Relationships to Optimise Manufacturing Operations
During CPHI Barcelona 2023, insightful content sessions offered attendees the chance to explore trending topics with expert speakers and panellists. Here, we summarise what the pharma industry and supply chain are talking about the most.18 Dec 2023
Biopharmaceuticals Products (378)
-
Product HMG/Menotrophin for Injection BP 75 IU -Humenotropin HP 75 IU
Human Menopausal Gonadotrophin 75 IU (lyophilized, sterile powder), & .0 ml Sodium Chloride Injection. This medication is used to treat certain fertility problems in women. It provides follicle stimulating hormone (FSH) and luteinizing hormone (LH) that help stimulate healthy ovaries to make eg...
-
Product FSH/Urofollitropin for Injection BP 75 IU - Ovitropin FSH 75 IU
Urofollitropin is a human follicle-stimulating hormone (FSH) analogue that is used to treat infertility in women. Available as combipack of Urofollitropin for Injection BP 75 IU & Sodium Chloride Injection BP 0.9%w/v

-
Product Mobius® Breez Microbioreactor System
The Mobius® Breez Microbioreactor is a 2 mL automated single-use perfusion cell culture platform designed to support gentle, adaptable, and reproducible cell processes. The system is capable of accelerating cell line development, media screening and optimization, and early process development
-
Product Protein stabilizer portfolio
Specifically developed for high-risk applications, our portfolio of high-quality sugars, polyols, amino acids, and surfactants simplifies selection when you face critical challenges such as preventing aggregation during manufacturing.
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product HEPAROSAN
Building on the success of hyaluronic acid, HTL now wants to bring heparosan to market. Heparosan is a promising new biopolymer for the design of innovative medical and pharmaceutical devicesHeparosan is a glycosaminoglycan, a natural biopolymer, produced by HTL Biotechnology from substances of non-animal ...
-
Product Life Cycle Services
Catalyx is a trusted partner in delivering world-class lifecycle services to regulated and high-risk end markets. With a relentless commitment to innovation and excellence, we partner with life science and other highly regulated organisations, to empower them to enhance efficiency, and drive success.&...
-
Product Peptide and Protein Technology
Almac’s peptide and protein technology offering is a key component within our suite of services. We offer a complete range of peptide and protein services from catalogue products through to GMP manufacture from early phase to commercial launch. We have a proven track record in high quality supp...
-
Product Biologic Quality Control and Release Testing
We deliver responsive QC analysis for complex biologic products from our cGMP laboratories. Our scientists develop and validate methods or perform technology transfer of a sponsor's method for a wide range of analytical methods required for batch release testing. We also routinely carry out testing to Phar...
-
Product Trypsin
Trypsin, a digestive proteolytic enzyme cleave peptide bonds specifically at the carboxyl end of the lysine and arginine residues.Recombinant trypsin, animal-derived component free, compliance with the pharmacopoeia standards,
We offers different formulations including lyophilizate and liquid pr...
-
Product Flexicon
At the heart of all the Flexicon filling systems is the low shear, gentle pumping action of our peristaltic fillers which ensure your valuable product is transferred undamaged with high accuracy and precision.
Flexicon offers a range of liquid filling and capping systems that gro...
-
Product human growth hormone(somatropin) rHGH
Recombinant Human Growth Hormone( r-hGH) Somatropin (rDNA Origin)
Molecular formula:C990H1528N262O300S7 Formula weight:22125D Category:White Lyophilized Powder
Indication:Pediatric growth hormone deficiency , Severe burns, Adult Growth hormone deficiency, Delay menopause, Bea...
-
Product Azelaic Acid
Usage:
1. Industrial:
(1)It is an additive in the production of complex lithium base grease;
(2)The raw material for the production of plasticizer;
(3)It can be used as an intermediate in some new coatings, polyurethane, nylon industry;
(4)It is a...
-
Product Primary Packaging for IV-solution bags
PolyCine offers:
Primary Packaging Films for IV-solution bag
Filling and transfer tubes for Primary Packaging
Hot Foils for IV-solution bag / primary container printing
Compounds for Primary Bag Connectors & Ports

-
Product Single-Use Assemblies
Whether you are looking for an economical standard single-use system or require a custom-designed and built single-use system, we can offer a proven solutions at every scale.
With a wealth of experience, knowledge, and solid quality processes behind every single-use product, we strive to assure ...
-
Product Acid red 87 (eosin y ws , c.i.45380)
Macsen laboratories offers a wide range of products which includes acid red 87 (eosin y ws, c.i.45380). It belongs to Speciality dyes and biological stains category. Applications: acid red 87 also known as eosine is used mainly as a dye for various industrial, cosmetic & scientific applications. Contact u...
-
Product Aramus™ 2D Bag Chambers from Entegris
These high-performance fluid storage and transfer bag chambers are made of a high-grade, gamma-stable fluoropolymer that provides high purity, exceptional compatibility and increased safety for critical process fluids and high-value final products. They also have a wide operating temperature range of -...
-
Product Mineral Phosphates
Dr. Paul Lohmann® offers a wide range of Mineral Phosphates for the Biopharmaceutical industry. They are widely used in various processes such as upstream, downstream and drug formulation and are an essential part of the entire biomanufacturing process. We also offer buffers as solid premixes or readily... -
Product NABOTA®
Botulinum toxin type A
Indication: Glabella Lines (Approved in KR, US, CA, EU), Post Stroke Upper Limb Spasticity, Crow's feet, Blepharospasm, Benign Masseteric Hypertrophy (Approved in KR)
- Nabota is the only 900kDa Neurotoxin approved in the US and EU since Botox.- It has demonstra...
-
Product Cell Culture Services
FUJIFILM Diosynth Biotechnologies is a leading provider of cell culture services for biologics, advancing anddelivering life changing therapies. We offer complete solutions for cell line development, process development, late phase activities, clinical and commercial manufacturing of a wide variety of biop...
-
Product 4000-LC Series Controller Rate Chamber
FARRAR™ pioneered forced air convection cooling to answer the challenge of preserving biologic samples and materials. The Controlled Rate Chamber (model 4000-LC series) features a 12-inch HMI screen with graphical display of chamber and product temperature. The unit provides repeatable, precise, ...
-
Product Sodium Hyaluronate - Medical grade
Hyature® is a pharmaceutical grade sodium hyaluronate which can be used as an API or excipient for drugs and medical devices in ophthalmic preparations, intra-articular injections, anti-adhesive preparations, topical preparations for wound healing and soft tissue filler, etc. Hyature® sodium hyalur...
-
Product Imipenem and cilastatin sodium
Imipenem and cilastatin sodium:
5kgs/aluminum tin; 2tins/carton;
-
Product Gene and Cell Therapy
Let’s light up your gene and cell therapy together.
Our Pfizer scientists and manufacturing experts are here to help you navigate the path to market for your gene and cell therapy
Experience is crucial when producing life-changing gene and cell therapies, so choosing the right CDMO is...
-
Product Telpegfilgrastim injection(PEG G-CSF)
Pegylated Human Granulocyte Colony-Stimulating Factor
Trade name: PEGNEUGEN
Technology
Recombinant gene technology, expressed by Yeast
Amino acid
175 amino acids; Non-glycosylated
...
-
Product GMP or non-GMP Contract Manufacturing
Actylis continues to expand its manufacturing capabilities by serving customers on a contract basis for exclusive manufacture of fine chemical compounds. We can provide a comprehensive tailor-made service to our clients, ensuring process development, optimization and commercial scale-up of processes are ca...
-
Product USP-NF, FCC, BP, EP, JP Chemicals
Spectrum Chemical offers the largest portfolio of USP-NF, FCC, BP, JP grade chemicals in the industry – more than 1,200 USP-NF chemicals including 700 in bulk sizes.
With our large portfolio, meet the monograph requirements specified in the USP and NF, which are the official standards for all pr...
-
Product Automatic Chromatography Column
Truking Technology Ltd provides wide range of pharmaceutical machineries which includes automatic dosing system. Features: with advanced processing equipment and mature production process, the main configuration (such as pipe fittings, pumps, valves, filters, instrumentation, plc, etc.) Are used world-clas...
-
Product 2023-Product Types
Product types covered by TPA members-Download our brochure or Contact [email protected] for more information
-
Product hyfc EPO (long acting)
Efesa, is a long-acting Erythropoietin indicated for CKD-induced anemia in dialysis and non-dialysis patients. Efesa is a hybrid Fc of IgG4 and IgD that provides hinge flexibility and long serum half-life without causing toxicity to target cells. Global Phase 3 trial for non-dialysis indication is currentl...
-
Product Aidaptus
Aidaptus has been awarded the Red Dot award for the category Product Design 2023
Benefits
- Aidaptus® is a 2-step single use auto-injector platform, with a versatile design and intuitive drug delivery.
- Aidaptus® accommodates both 1mL and...
-
Product Wireless Glove Integrity Tester GIT WLAN
• Data are wirelessly transmitted to the PC • In-situ testing without removal of the gloves • Multiple gloves can be tested simultaneously • Test reports are automatically generated • RFID chipset, recognize glove number automatically • Built-in battery and pump, no need for external air so...
-
Product Suitable Supports
Offering Tailor-made catalysts for optimum productivity in your process.
Hindustan Platinum is a leading manufacturer of precious metal catalysts that play a pivotal role in chemical industries, owing to their activity, selectivity and recyclability. Our catalysts are abundantly suppli...
-
Product Primary API reference standards
Mikromol reference standards for Active Pharmaceutical Ingredients (APIs) are primary quantitative standards – the majority accredited to ISO 17034 and designed to ensure the highest analytical accuracy and reliability. Their intended use is for potency assessments, drug substance and drug product release ...
-
Product BioHale® Sucrose
BioHale® Sucrose is a non-reducing crystalline disaccharide made up of glucose and fructose. Sucrose is commonly used in the biopharmaceutical industry to stabilize proteins, lipids, and carbohydrates during the formulation process. The utility and function is driven by its unique chemical and physical pro...
-
Product Cappwash Plate Washer by CAPP
is an ideal elisa plate washer for small scale work. available in an 8-channel, a 12-channel and a 16-channel version. CAPP elisa plate washer requires no programming nor electrical wiring connections.
It is made of a high-grade stainless steel and polypropylene, making it fully aut...
-
Product Biopharmaceutical development
From initial characterization of drug substance to commercial batch release, each phase of your drug product development requires analytical methods to qualify the product for safety, integrity, strength, purity, and quality. With these critical attributes in mind, Alcami’s biologics analytical devel...
-
Product Nitrosamine Impurities Testing
SGS Life Sciences has considerable expertise in the method development of nitrosamine determination in pharmaceutical products. SGS has established a specific method for NDMA which can be applied to various different matrices. Alternatively, a platform method, based on trace-level detection by LC-MSMS, is ...
-
Product Chromalite synthetic chromatograhy resins
Analytical & preparative bio-chromatographic ResinsPurolite Chromalite chromatography resins are versatile bulk processing media designed especially for large-scale chromatography applications. Purolite Chromalite chromatography resins are available for hydrophobic interaction (HIC) and ion exchange (I...
-
Product Octreotide
Octreotide is the acetate salt of a cyclic octapeptide. It is a long-acting octapeptide with pharmacologic properties mimicking those of the natural hormone somatostatin. Prevention and treatment of acute pancreatitis and upper gastrointestinal bleeding, and can also be used for portal hypertension a...
-
Product EPOETIN - ERYTHROPOIETIN - HEMAX / HYPERCRIT / EPOYET
Freeze dried vials containing 1K IU, 2K IU, 3K IU, 4K IU, 10 K IU; 20K IU; 40K IU
-
Product (6-Aminopyridin-3-yl)boronic acid hydrochloride
CAS NO.:1309982-15-7Appearance:Molecular Formula:C5H8BClN2O2Formula Weight:174.39
-
Product HBTU; CAS#94790-37-1 Peptide Coupling Reagent
Coupling Reagents of peptides; Purity(HPLC): 99% min;
-
Product AdvantaPass®
AdvantaPass® pass through technology permits the aseptic transfer of fluids between the walls of pharmaceutical manufacturing suites. It’s the first system of its kind to offer complete isolation between different classes of clean rooms when transferring multiple lines of fluid through a single stainl...
-
Product Caffeic acid
Synonyms: 3,4-Dihydroxycinnamic acid, 3,4-dihydroxybenzeneacrylic acid
CAS: 331-39-5
Molecular Formula: C9H8O4
Molecular Weight: 180.16
Properties: Light yellow to yellowish brown crystal; slightly soluble in water, easy to dissolve in hot water, cold ethanol, an...
-
Product RoSS.FRDG: Ultra Cold Storage
• Insulated container for long-term cooling of drug substances in RoSS Shells • Upright Freezer Style • Ultra-cold Freezer: Holds desired temperature down to -80°C • Ease of use for loading: place loaded Trolleys or Racks as a whole inside • Fully modular: customized shelving systems • GMP compliant ...
-
Product INJECTA 36 - High-speed, advanced robotic processing for Ready-To-Use syringes
Enhancing the performance of advanced robotics, the INJECTA 36 raises the bar for high-speed production of Ready-To-Use syringes and pre-capped cartridges. The same cutting-edge robotic technologies assure accurate no-touch-transfer component processing with minimal operator access to the working area....
-
Product Nitrogenema II
This enclosure is capable of protecting your process by nearly eliminating moisture or oxygen with the flow of inert gas. Don’t spend tens of thousands of dollars on a cumbersome metal isolator, see how the Flow Sciences END Series can solve your application needs.
Achieve and maintain low humidity ...
-
Product Extractables & Leachables Testing
The assessment of Extractables and Leachables in bio/pharmaceutical products is an important step in drug product development. Processing equipment, as well as, primary and secondary container closures are potential vectors for chemical contaminants. Monomers and polymer additives such as antioxidants, pla...
-
Product Pyrogen free chondroitin sulphate
Syntex manufactures a wide range of active pharmaceutical ingredients, including sodium heparin both from bovine and porcine origin, calcium heparin, lithium heparin, low molecular weight heparin and pyrogen free chondroitin for injectables. Syntex complies with GMP and exports to Annex I countries, such u...
-
Product 1,2-Ethanedithiol
GL Biochem (Shanghai) Co., Ltd offers a wide range of reagents which includes 1,2-Ethanedithiol. Contact us for more information.
-
Product Beer Yeast Series
Angel yeast co.,ltd provides wide range of biotechnology products which includes beer yeast series. Contact us for more information.
-
Product 1-pentanesulfonic acid sodium salt monohydrate 25g/100g ethane-1,2- sulfonic acid double sodium salt 5g
Jiangsu Hanbon Science And Technology Co. Ltd offers wide range of chemical reagent which includes 1-pentanesulfonic acid sodium salt monohydrate 25g/100g ethane-1,2- sulfonic acid double sodium salt 5g . Contact us for more information.
-
Product Adenosine injection
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes adenosine injection. It belongs to bio-products category. Contact us for more information.
-
Product 1,500iu tetanus antitoxin
Jiangxi institute of biological products offers bio products which includes 1,500iu tetanus antitoxin. Features: it is clear, colorless or pale yellow liquid. It is first choice for prophylaxis and therapy of tetanus. Contact us for more information.
-
Product Solid state chemistry
Wilmington pharma tech co llc offers a wide range of services which includes solid state chemistry. Features: it includes salt screening and polymorph screening. Contact us for more information.
-
Product HCG/Chorionic Gonadotrophin for Injection -OVIGIL HP 5000 IU
Human chorionic gonadotropin (HCG), lyophilized powder, vial of -5,000 IU is a highly purified pyrogen-free preparation obtained from the urine of pregnant females a hormone that supports the normal development of an egg in a woman's ovary, and stimulates the release of the egg during ovulation.
-
Product Viscosity Reduction Platform
The Viscosity Reduction Platform of proprietary excipient combinations enables subcutaneous formulation of highly concentrated protein biologics, bringing together increased concentrations and improved downstream processing.
-
Product Deviron® C16 Detergent EMPROVE® EVOLVE
Deviron® C16 detergent provides robust inactivation of enveloped viruses. In addition, it may be used to achieve cell lysis for viral gene therapy vector production processes. It does not require a REACH authorization and is a suitable alternative to Triton™ X-100 detergent.

-
Product Benzonase® endonuclease portfolio
Benzonase® endonuclease is a unique, genetically engineered endonuclease that offers a variety of advantages over existing methods of nucleic acid removal. This enzyme has been designed to be supplied without protease activity and without the viral contaminants that can accompany enzymes isolated from ...
-
Product Functionalized Hyaluronic Acid
HTL Biotechnology offers expert guidance and support to companies venturing into the industrial production of a modified Hyaluronic Acid compound, ensuring transparent and efficient processes.
New functionalities thanks to chemical modification:
Optimizing ...
-
Product Catalyx SmartFactory Machine Vision
Our machine vision inspection systems are designed to enhance the efficiency and accuracy of laboratory processes, reduce the reliance on manual inspection, and minimize the risk of human error. Our solutions are ideal for laboratories seeking to streamline their workflows, improve quality control, and acc...
-
Product Control System Integration
Control System Integration
Catalyx designs automation systems to deliver integrated, comprehensive, and specialized connectivity solutions, networks, and digital ecosystems to organizations around the world. By working at all stages in the project lifecycle – from Inception and Front-End Enginee...
-
Product Monitoring, Notifications, and Alarm Integration
Catalyx is experienced in deploying Monitoring and Notification Systems across a wide variety of industries, bringing cutting-edge technologies for a flexible and customized yet standardized solution. We have developed sophisticated EMS and BAS systems with Alarm Management, including user-based hierarchy ...
-
Product Quality and Compliance
Quality and Compliance
Catalyx can help keep your operations safe and compliant. We bring extensive process industry expertise and provide a variety of automation regulatory system services as part of our Automation Solutions offering — delivering integrated process compliance systems and regula...
-
Product Catalyx SmartFactory Robotics
Catalyx SmartFactory Robotics
Catalyx specializes in the comprehensive design, development, integration, qualification, and ongoing support of automated robotic and cobot systems in lab environments. Catalyx offers cutting-edge robotic material handling solutions and specializes in crafting cust...
-
Product Catalyx OpenBIO® Bioreactor Product Family
Catalyx OpenBIO® Bioreactor Product Family
The OpenBIO Product Family is designed to be the high functioning centerpiece of your laboratory, process development, and manufacturing operations. With a focus on recipe-based operations and connectivity, scaleup and tech transfer is v...
-
Product Analytical Services
Our state-of-the-art analytical testing labs support drug substance (API) and drug product (finished product) analytics across all phases of clinical development and into commercial release.
We employ over 170 highly skilled analysts working in GMP environments across the UK, Europe, and the...
-
Product NeoPeptides™
Our NeoPeptide™ experts have been associated with the individualised cancer vaccine field for several years and our MHRA registered facility and systems have been established to enable fully GMP compliant manufacture and release of 20-30 GMP peptides within less than three weeks. A bespoke Pharmaceutical Q...
-
Product Physical Sciences
We are experts in solid state chemistry.Through the creation of synergy between solid state chemistry and formulation development, our experienced scientists have the ability to
-
Product Biosimilars Testing Services
Testing services for biosimilars: Our biosimilar analytical comparability programs provide highly relevant data for early stage characterisation and later stage comparison. These programs evaluate and compare all pertinent features of the biosimilar product and are based on the ICH Q6B. Programs encompa...
-
Product Antibody / Monoclonal antibody therapeutics services
Monoclonal antibody therapeutics: We continually invest in advanced analytical instrumentation that allows us to deliver mAb analytical data with the highest degree of sensitivity, accuracy and resolution. Our experience spans recombinant monoclonal antibodies and related products such as biosimilars, ...
-
Product Protein Aggregation Analysis and Characterization
Analysis and characterization of protein aggregates: The services provided by our experts include a range of protein aggregation analysis techniques that are used to detect and quantify aggregates in solution in support of formulation development, quality control, stability studies, comparability, rel...
-
Product Biopharmaceutical Characterisation and Testing Services
Characterization and testing of biopharmaceuticals: Using our expert's insight into a biologic's structure, physicochemical properties, potency, and impurity profile, we help you meet your development milestones.
A range of expertise is available, including assessment of structure...
-
Product cGMP Cell-based Bioassays
Cell-based bioassays that comply with cGMP: Using a variety of techniques, including cell-based assays, ligand and receptor binding assays, and flexible multiple assay approaches – our potency testing experts offer tailored bioassay method development, method transfer and method validation to ICH Q2 (R1)...
-
Product Bioanalytical Services (GLP/GCP)
Services for Bioanalysis (GLP/GCP): In our state-of-the-art facilities, we provide GLP/GCP method development, validation, method transfer, sample analysis and pharmacokinetic and toxicokinetic support, along with automated data collection and reporting systems. Over the past 20 years, we have supported...
-
Product Bispecific Antibody Characterisation
Characterisation of bispecific antibodies supporting regulatory considerations for therapeutic development. Our scientists offer BsAb characterisation, with a focus on monitoring relevant CQAs. We can help you to demonstrate consistency or comparability of manufactured batches or as release tests for clin...
-
Product Analytical Research and Development Services
Services for analytical research and development: As your organization focuses on core business goals, you'll require a partner who has a history of providing consistently high-quality regulated compliance testing. Our team are focused on supporting product development, GMP manufacturing and distributio...
-
Product Hyaluronidase
Hyaluronidase, an enzyme that breaks down hyaluronic acid, has long been used to facilitate the dispersion and absorption of other drugs and to reduce tissue damage in cases of extravasation of a drug. Recombinant human hyaluronidase(rhPH20)developed by BaoPharma(Shanghai)...
-
Product Chymotrypsin
Chymotrypsin, a digestive proteolytic enzyme cleave peptide bonds formed by the carboxyl groups of Tyr, Phe, Trp, which has been in clinical use since the 1960s. It provides better resolution of inflammatory symptoms and promotes speedier recovery of acute tissue injury than several of the ot...
-
Product Collagenase
Collagenase,break down the native collagen that holds animal tissues together and, are made by a variety of microorganisms.and by many different animal cells.
Recombinant Collagenase of Clostridium histolyticum developed by BaoPharma(Shanghai) is a chromatographically purified ...
-
Product Cartridges
Masterfilter produces reliable and high-quality filter cartridges for the microfiltration of liquids and gases. Various materials such as Activated Carbon, Stainless Steel, Glass Fiber, Resin-bonded Fibers, Nylon, PES, PP, PTFE and PVDN are available as filter media with micron ratings from 0.01 to 150 µm.
-
Product Capsules
Masterfilter produces reliable and high-quality filter capsules with pleated membranes for the microfiltration of liquids. Various filter media such as Glass Fiber, Nylon, PES, PP, PTFE and PVDN with micron ratings from 0.2 to 10 µm are available.
-
Product L-Tyrosine Disodium Salt 2-hydrate
Dr. Paul Lohmann® offers a wide range of high-quality Mineral Salts in upgraded purity for all bioproduction streams. Highlighted are the sequestering Disodium EDTA 2-hydrate, the nutrient L-Tyrosine Disodium Salt 2-hydrate and the pH regulating Sodium Succinate 6-hydrate. Please contact ... -
Product 4000 Series Controller Rate Chamber (Standard)
FARRAR™ pioneered forced air convection cooling to answer the challenge of preserving biologic samples and materials. The Controlled Rate Chamber (model4000 series) provides repeatable, precise, and rapid freezing and thawing of bulk materials and sample prior to cold storage. This precision controlled rap...
-
Product ULC Series 311
FARRAR™ pioneered forced air convection cooling to answer the challenge of preserving and storing biological and human tissue donor samples and materials. Purpose-built for life science applications, the Ultra-Low Chamber ULC-190, new ULC-259, andULC-311 are the only +2°C to +8°C and -20°C to-80°C, forced ...
-
Product ULC Series 190
FARRAR™ pioneered forced air convection cooling to answer the challenge of preserving and storing biological and human tissue donor samples and materials. Purpose-built for life science applications, the Ultra-Low Chamber ULC-190, new ULC-259, andULC-311 are the only +2°C to +8°C and -20°C to-80°C, forced ...
-
Product Hyatrue® — Pharmaceutical Grade Sodium Hyaluronate
Hyatrue® is a pharmaceutical grade sodium hyaluronate which can be used as an API or excipient for drugs and medical devices in ophthalmic preparations, intra-articular injections, anti-adhesive preparations, topical preparations for wound healing and soft tissue filler, etc. Hyatrue® sodium hyaluronate ...
-
Product Oetiker® Securing Clamps
Qosina offers an extensive selection of Oetiker® clamps that can be used in a wide range of applications where robust, secure connections are required.
Qosina’s line of Oetiker ear clamps range in size from 0.114 inch closed - 0.146 inch open range (2.9 mm - 3.7 mm) to 1.547 inch closed ...
-
Product OneShot™ Single-Use Filling Needles from Overlook Industries
OneShot™ single-use filling needles help boost production by reducing filling line downtime and eliminate the risk of cross-contamination and the need for costly cleaning and validation.
The combination of plastic and stainless steel allows customers to experience the benefits of disposability whi...
-
Product Mipeginterferon alfa-2b
Pegylated human interferon alfa-2b
Trade name: PEGBING
Technology
Recombinant gene technology, expressed by Yeast
Amino acid
165 amino acids
Molecular weight
Approximately 59 kD
Indications
Chronic hepatitis B & C
...
-
Product Oprelvekin IL-11
Human Interleukin 11
Trade name: TOPMEGA
Technology
Recombinant gene technology, expressed by E.coli
Amino acid
177 amino acids; Non-glycosylated
Molecular weight
Approximately 19.0kD
Indications
Thrombocytopenia
Administration
s....
-
Product Analytical Services
Actylis performs routine and non-routine analytical testing for various industries, in support of manufacturing processes, QA/QC functions, R&D projects, and environmental applications. Leveraging state-of-the-art instrumentation, extensive knowledge base, as well as internal, cross-functional competen...
-
Product 2023-Dosage Forms
Dosage forms covered by TPA members-Download our brochure. For more information, please contact [email protected]
-
Product Nimotuzumab
Nimotuzumab binds with intermediate affinity and high specificity to the extracellular domain of EGFR and blocks the binding of the EGF to its receptor and inhibits tumor cell growth. The molecule induces cell lysis through ADCC mechanism. Nimotuzumab provides vaccinal effect that induce memory T-cell ...
-
Product Bevacizumab
Bevacizumab acts by selectively binding circulating VEGF, thereby inhibiting the binding of VEGF to its cell surface receptors. This inhibition leads to a reduction in microvascular growth of tumor blood vessels that limits the blood supply to tumor tissues.The indication is for Colorectal Cancer...
-
Product Erythropoietin Alpha
Indication: Chronic kidney diseases induced anemia (dialysis and non-dialysis)

-
Product Insulin Glargine
Long-acting basal insulin analogue to treat Type I and II Diabetes Mellitus.
-
Product Homogeneous Catalyst
Hindustan Platinum leads the way by providing a complete loop for recovery of PGM from spent homogeneous catalysts.
-
Product Salts & Solutions
Offering Tailor-made catalysts for optimum productivity in your process.
Hindustan Platinum is a leading manufacturer of precious metal catalysts that play a pivotal role in chemical industries, owing to their activity, selectivity and recyclability. Our catalysts are abundantly su...
-
Product Impurity reference standards (including a broad range of degradation products)
Impurities are always present in a therapeutic substance and can significantly alter a drug’s effects on the patient, potentially putting their health at risk. Legislators and official bodies, therefore, set limit and threshold values as well as guidelines requiring the detection, identification, quantific...
-
Product Reference Materials Management
To support you in bringing even safer medicines to the market, we go far beyond supplying you with the right reference standards. From impurity profiling and working standards outsourcing services, all the way to managing inventory and logistics of your reference standards, we’re fully equipped to provide ...
-
Product Excipient and Concomitant component standards
Designed to deliver the highest accuracy and reliability, Mikromol excipient standards are primary quantitative standards that are suitable for assay development or as working standards. Most of the 170+ excipient products in our range are ISO 17034 accredited, and all are supplied with detailed COAs featu...
-
Product Custom reference standards
Creating pharmaceutical reference standards to customer specification has always been at the heart of the Mikromol business. We understand that you are constantly discovering new actives and impurities of interest – each year, our dedicated, highly-qualified customs team produces several hundred new materi...
-
Product BioHale® Trehalose Dihydrate
BioHale® Trehalose Dihydrate is a non-reducing disaccharide consisting of two glucose molecules which are linked by an α, α-1,1 bond. It is used in the pharmaceutical industry as a stabilizer of biologics.
Trehalose Dihydrate has proven to be highly effective in protecting cell membranes...
-
Product 1,3,5-Benzenetricarboxylic acid chloride 4422-95-1
Appearance:Colorless or light yellow solidAssay:99%min
-
Product Potassium metavanadate 13769-43-2
Appearance:White powderAssay(V2O5):65%min
K2O:33%min
Cr:0.05%max
Na:0.5%max
Fe:0.05%max
Cl:0.01%max
As:0.005%max
-
Product 1-Hydroxy-7-azabenzotriazole 39968-33-7
Appearance:Off-white to white crystallinePurity (HPLC):99%min
Melting point:213.0 ~ 216.0 oC (dec.)
Loss on drying:0.5%max
-
Product Proteinase K 39450-01-6
Appearance: white solid Protein purity:95%minSpecific activity:30.0U/mg P min
Rnase activity: none detected
Dnase activity: none detected
-
Product Biosafety Testing
Life-saving medicines are heavily regulated during development, manufacture and distribution. To fulfill regulatory requirements, the biopharmaceutical industry is increasingly looking for independent service providers who can deliver comprehensive characterization solutions on one site.
SGS’s glo...
-
Product Quality Control Testing
Bringing a compound from the laboratory to the market is a long and winding road with a great number of scientific, safety and regulatory challenges. SGS has been offering high quality analytical testing and clinical research services to support drug research, registration and production.
SGS leve...
-
Product Viral Safety Testing
SGS’s global center of excellence for cell bank characterization & virus testing is located in the United Kingdom and provides services with ultimate reliability, highest GLP/cGMP quality & scientific expertise.
For any of your biologics, we help you comply with the global regula...
-
Product Praesto Agarose Chromatography resins
Ion exchange chromatography (IEX) separates proteins and other biomolecules based on their charge. IEX resins have charged functional groups that bind molecules with an opposite charge. Bound molecules are most commonly eluted by an ascending salt gradient. Alternatively, a shift in pH that changes...
-
Product Bivalirudin
Use as an anticoagulant in patients with unstable angina undergoing percutaneous transluminal coronary angioplasty .Bivalirudin is a synthetic 20 residue peptide (thrombin inhibitor) which reversibly inhibits thrombin. Once bound to the active site, thrombin cannot activate fibrinogen into fibrin, t...
-
Product Liraglutide
Diabetes mellitus,
Liraglutide is a once-daily GLP-1 derivative for the treatment of type 2 diabetesLabel,2. The prolonged action of liraglutide is achieved by attaching a fatty acid molecule at position 26 of the GLP-1 molecule, enabling it to bind reversibly to albumin within the subcutaneou...
-
Product SOMATROPIN - HHT
Freeze dried powder for reconstitution containint Somatropin 4 IU or Somatropin 16 IU
-
Product FILGRASTIM - NEUTROMAX
SOLUTION FOR INJECTION. Vials containing filgrastim 300 µg (30 M IU) or 480 µg (48 M IU)
-
Product ((1S,4R)-4-(6-aMino-9H-purin-9-yl)cyclopent-2-enyl)Methanol
CAS NO.:147332-45-4Appearance:White powder Molecular Formula:C11H13N5O Formula Weight:231.25
-
Product (+)-JQ-1
CAS NO.:1268524-70-4Appearance:White powder Molecular Formula:C23H25ClN4O2S Formula Weight:456.14
-
Product DSC; CAS# 74124-79-1 Peptide Coupling Reagent
Coupling Reagents of peptides; Purity(99% HPLC): 99%min;
-
Product NHS/ HOSU; CAS#6066-82-6 Peptide Coupling Reagent
Coupling Reagents for peptides ; it is also analytical chemistry; Purity(HPLC): 99%min;
-
Product WMArchitect
Custom single-use assemblies designed for your bioprocess
puresu® is a combination of different components that can be customised to your requirements. Through a broad range of tubing and single-use components combined across an open architecture design, we wo...
-
Product Watson Marlow Pump
Developed for application requirements, our range of peristaltic pumps offer an industry-leading solution to fluid handling challenges. Leveraging over 60 years of pump engineering experience, we are continuously working to innovate the latest technologies in fluid management.
Backed by a global...
-
Product Watson Marlow Tubing
Watson-Marlow Tubing was born out of a desire to optimise the performance of our industry-leading pumps. Leveraging decades of experience in peristaltic technology development, our tubing products pair with our pump portfolio to offer a complete solution to our customers.
Since 2000, Watson-Marl...
-
Product AFLEX
For over 40 years, Aflex has been producing the most technically advanced range of PTFE-lined flexible hose products in the world.
From our factories in the UK and USA, we design, develop and manufacture our hoses from raw materials to finished products. This comprehensive approach gives us an...
-
Product Oetiker® Tubing Connections Kit - Q6000 OET
Qosina now offers an Oetiker® tubing connections kit that is perfect for companies or universities performing single-use training or looking to demonstrate how Oetiker clamps are connected. Kit contents are noted below and include components that allow for connection of five Oetiker clamp assemblies as...
-
Product Microbial Services
We are a world-leader in process development and cGMP manufacturing of proteins derived from a range of microbial hosts including E. coli, P. pastoris and S. cerevisiae .
-
Product ULC Series 259
FARRAR™ pioneered forced air convection cooling to answer the challenge of preserving and storing biological and human tissue donor samples and materials. Purpose-built for life science applications, the Ultra-Low Chamber ULC-190, new ULC-259, andULC-311 are the only +2°C to +8°C and -20°C to-80°C, forced ...
-
Product Molgramostim(GM-CSF)
Human Granulocyte Macrophage Colony-Stimulating Factor
Trade name: TOPLEUCON
Technology
Recombinant gene technology, expressed by E.coli
Amino acid128 amino acids; Non-glycosylated
Molecular weight
Approximately 14.6 kD
Indications
Neutropen...
-
Product Filgrastim(G-CSF)
Human Granulocyte Colony-Stimulating Factor
Trade name: TOPNEUTER
Technology
Recombinant gene technology, expressed by E.coli
Amino acid
175 amino acids; Non-glycosylated
Molecular weight
Approximately 18.8 kD
Indications
Neutropenia
qq...
-
Product IRIS SINGLE-USE ASSEMBLIES
You have the choice of putting together your single-use manifold assembly using the online configurator. Alternatively, you may select a preset designed by our experts and receive additional advice on it. In both events, our manifolds are double-fixed with ties and gamma irradiated or optionally e-be...
-
Product Lyomax
LYOMAX, Industrial Freeze Dryer
The freeze-drying process of today demands increased process control, with reduced processing time augmented with upgrades in equipment to remain compliant with a changing regulatory landscape. To accommodate such growth, IMA LIFE uses its in-depth process...
-
Product Vega
VEGA: Rotary washing machine
Vega is a washing machine for bottles, vials, ampoules and unstable cylinder-shaped containers. Born to satisfy the most stringent market requirements, it is available in four different models featuring a continuous rotary motion to reach very high speeds while...
-
Product Sensitive AP400 CW
SENSITIVE AP400 CW
Carton labelling machine with 100% check weighing system
The SENSITIVE AP400 CW is one of the latest developments in the field of carton labelling. Integrated with a 100% check-weighing system, it is conceived to apply labels onto three of the carton’s sid...
-
Product Xtrema
XTREMA, High Speed Filling and Stoppering Machines for Aseptic Environments
XTREMA fundamental characteristics respond to the industry’s stringent technical requirements of vial filling, and with its flexible and ergonomic design it assures the most complete possibility to integrate mod...
-
Product Biologics Characterization
SGS’ range of services dedicated to biopharmaceutical product characterization bring the most recent developments in testing technology to companies across the globe. In 2010, SGS acquired the M-Scan Group, the world leaders in the application of advanced mass spectrometry
techniques for protein and c...
-
Product Biologics Characterization Services
As a pioneer of physicochemical characterization, we offer you unrivaled expertise in protein analysis. Our laboratories helped to develop mass spectrometry (MS) mapping of biotechnology products, together with other mass spectrometry strategies related to protein/glycoprotein analysis, which have become s...
-
Product Interferon beta 1a (IM) - ESCLEROFERON
Ready to use PFS containing Interferon Beta 1a for intramuscular injection. Each PFS contains interferon Beta 1a 30 µg
-
Product (R)-1,3-Dimethyl-piperazine dihydrochloride
CAS NO.:1152110-26-3Appearance:Off-white powder Molecular Formula:C6H16Cl2N2 Formula Weight:186.07
-
Product (2S)-2-(2-Oxopyrrolidin-1-yl)butanoic acid
CAS NO.:102849-49-0Appearance:Light yellow powder Molecular Formula:C8H13NO3 Formula Weight:171.19 Melting Point:118-121°C
-
Product TCEP.HCL; CAS#51805-45-9 Peptide Crosslinking Reagent
Crosslinking Reagents, this reagent can used in the reduction of disulfide bonds.Assay(AT): 98%min.
-
Product Calcium heparin
Syntex manufactures a wide range of active pharmaceutical ingredients, including sodium heparin both from bovine and porcine origin, calcium heparin, lithium heparin, low molecular weight heparin and pyrogen free chondroitin for injectables. Syntex complies with GMP and exports to Annex I countries, such u...
-
Product Lithium heparin
Syntex manufactures a wide range of active pharmaceutical ingredients, including sodium heparin both from bovine and porcine origin, calcium heparin, lithium heparin, low molecular weight heparin and pyrogen free chondroitin for injectables. Syntex complies with GMP and exports to Annex I countries, such u...
-
Product Low molecular weight heparin
Syntex manufactures a wide range of active pharmaceutical ingredients, including sodium heparin both from bovine and porcine origin, calcium heparin, lithium heparin, low molecular weight heparin and pyrogen free chondroitin for injectables. Syntex complies with GMP and exports to Annex I countries, such u...
-
Product Sodium heparin for injectables
Syntex manufactures a wide range of active pharmaceutical ingredients, including sodium heparin both from bovine and porcine origin, calcium heparin, lithium heparin, low molecular weight heparin and pyrogen free chondroitin for injectables. Syntex complies with GMP and exports to Annex I countries, such u...
-
Product 1H-Tetrazole
GL Biochem (Shanghai) Co., Ltd offers a wide range of reagents which includes 1H-Tetrazole. Contact us for more information.
-
Product Aclohol Yeast Series
Angel yeast co.,ltd provides wide range of biotechnology products which includes aclohol yeast series. Contact us for more information.
-
Product Nutrient enriched yeast extract
Angel yeast co.,ltd provides wide range of biotechnology products which includes nutrient enriched yeast extract . Contact us for more information.
-
Product Autolyzed yeast & Inactive yeast
Angel yeast co.,ltd provides wide range of biotechnology products which includes autolyzed yeast & inactive yeast. Contact us for more information.
-
Product Atorvastatin Intermediates
Angel yeast co.,ltd provides wide range of biotechnology products which includes atorvastatin intermediates. Contact us for more information.
-
Product 1-hexanesulfonic acid sodium salt 25g/100g lauryl sodium bisulfate salt 5g/25g
Jiangsu Hanbon Science And Technology Co. Ltd offers wide range of chemical reagent which includes 1-hexanesulfonic acid sodium salt 25g/100g lauryl sodium bisulfate salt 5g/25g . Contact us for more information.
-
Product 1-octanesulfonic acid sodium salt 25g/100g tetraethyl ammonium bisulfate 5g/25g
Jiangsu Hanbon Science And Technology Co. Ltd offers wide range of chemical reagent which includes 1-octanesulfonic acid sodium salt 25g/100g tetraethyl ammonium bisulfate 5g/25g . Contact us for more information.
-
Product 25g/100g tetrabutyl ammonium bisulfate 5g/25g
Jiangsu Hanbon Science And Technology Co. Ltd offers wide range of chemical reagent which includes 25g/100g tetrabutyl ammonium bisulfate 5g/25g . Contact us for more information.
-
Product 1-decanesulfonic acid sodium salt 25g/100g g cetyl trimethylammonium hydrogen sulfate 5g/25g
Jiangsu Hanbon Science And Technology Co. Ltd offers wide range of chemical reagent which includes 1-decanesulfonic acid sodium salt 25g/100g g cetyl trimethylammonium hydrogen sulfate 5g/25g . Contact us for more information.
-
Product Palladium diammine dichloride
Hindustan Platinum provides wide range of precious metals salts and solutions which includes palladium diammine dichloride. Contact us for more information.
-
Product Single Use Molded Manifolds
Single-use molded manifolds are custom made to meet your product sampling and storage needs in drug and vaccine production.
Manufactured from AdvantaFlex® biopharmaceutical grade TPE or AdvantaSil® platinum-cured Class VI silicone, molded manifolds eliminate the need for barb...
-
Product 10,000iu tetanus antitoxin
Jiangxi institute of biological products offers bio products which includes 10,000iu tetanus antitoxin. Features: it is clear, colorless or pale yellow liquid. It is first choice for prophylaxis and therapy of tetanus. Contact us for more information.
-
Product Pharmaceutical Product Testing
Element’s pharmaceutical laboratories provide specialist pharmaceutical testing services, including chemical and physical characterization, formulation development, microbial, stability and elemental impurity testing on a wide range of products, from raw materials to finished products.
-
Product Technology Transfer-Vaccines
We collaborate with over 20,000 partners eager to initiate technology transfer projects. In particular, we have a strong presence in the vaccine sector. If you're interested in developing vaccine products, we can connect you with the most suitable partners worldwide.
-
Product 2D Pillow Bag Solutions
Lightweight, hygienic and ergonomic 2D bag handling solutions. Maximise fluid recovery with our large range of easy handling products.
All of our products are customisable to individual requirements and specifications.
Configurations available to fit single use bags from all suppliers.&nb...
-
Product Single-Use Assemblies
Custom-engineered and overmolded assemblies best-suited for single-use applications.
-
Product Botulinum toxin type A 100 units (Hitox)
Botulinum toxin / Clostridium Botulinum Toxin Type A / 100Unit
Vacuum drying
Storage: 2~8℃

-
Product Insulin Aspart API
Tonghua Dongbao Group Import & Export Co.,Ltd.(Previously used name: Tonghua Dongbao Imp &Exp Co., Ltd.)was established in 1993 and is a wholly owned subsidiary of Dongbao Group. As the main body of Dongbao Group's overseas affairs, it is the central management department of Dongbao Group's f...
-
Product Insulin Glargine API
Tonghua Dongbao Group Import & Export Co.,Ltd.(Previously used name: Tonghua Dongbao Imp &Exp Co., Ltd.)was established in 1993 and is a wholly owned subsidiary of Dongbao Group. As the main body of Dongbao Group's overseas affairs, it is the central management department of Dongbao Group's f...
-
Product Insulin Degludec API
Tonghua Dongbao Group Import & Export Co.,Ltd.(Previously used name: Tonghua Dongbao Imp &Exp Co., Ltd.)was established in 1993 and is a wholly owned subsidiary of Dongbao Group. As the main body of Dongbao Group's overseas affairs, it is the central management department of Dongbao Group's f...
-
Product Distreptaza
Rectal suppositories indicated for:
1) Supportive treatment of pelvic inflammatory disease (PID) – inflammatory diseases of the ovaries, fallopian tubes and endometrium,
2) Adhesions after abdominal surgical procedures,
3) Acute haemorrhoidal disease and chronic haemorr...
-
Product Polyethylene Glycol Recombinant Human Growth Hormone Injection
Commercialized in China since 2014
Administration once a week

-
Product Recombinant Human Follicle-stimulating Hormone for Injection
Launched on the market since 2015.
-
Product Recombinant Human Granulocyte/Macrophage Colony-stimulating Factor Hydro-gel for Topical Application
Topical rhGM-CSF gel formulation made by patented E.coli secretion technology
-
Product Natural Vitamin
New Project Products:
Vitamin K2, Vegan Capsules, Methyl Folate, 25-Hydroxy Vitamin D3, Enzymes, Carotenoids, Collagen Peptides, Microencapsulated/Coated......
-
Product Single Use Bags
BioPac Single-use 2D & 3D bags are pre-sterilized customized products to meet your specific needs of storage, transfer and processing of media, buffers and intermediate fluids. Offered in sizes ranging from 10 mL to 1000 L
-
Product BIOPRODUCTION CONFERENCE - EVENT
3th & 4th of Avril 2024
Congress Centre, PARIS (France)
The event will bring together 550 decision-makers and stakeholders of the pharmaceutical bioproduction. It will last two days during which will be presented conferences, round tables, pitches, workshops and time...
-
Product AIRFREIGHT WITH GDP ACTIVE AND PASSIVE COLD SOLUTIONS
As IATA cargo agents, and GDP certified we offer direct services with the main airlines allowing us to offer the best air cargo, EXPORT and IMPORT and Cross-Trade rates, providing end-to-end logistics solutions from/to anywhere in the world.
We have air consolidation services and Chartering services. ...
-
Product CUSTOMS CLEARANCE & CONSULTANCY
Specialists in sanitary, veterinary, S.O.I.V.R.E., phytosanitary and pharmaceutical procedures.
Our specialists also offer their services in the control of goods in transit or any other customs situation (DA, DDA, ATA).
Consulting and Customs Compliance, tariff classification, Obtaining authoriza...
-
Product Labeesity®
Labeesity SFK7® is a standardized extract derived from Labisia pumila, a traditional herb native to Southeast Asia. It has gained recognition as a potential dietary ingredient due to its various health benefits. Notably, SFK7® has obtained approval as a New Dietary Ingredient from the US Food and Drug Admi...
-
Product Pervira®
Pervira® is a standardized extract derived from Kaempferia parviflora, a plant known for its medicinal properties. One of the key active components in Pervira® is 5,7-Dimethoxyflavone (5,7-DMF), which is present in a concentration of not less than 8%. The extract undergoes a standardized extraction pr...
-
Product Starpril®
Starpril® is a standardized extract derived from Orthosiphon stamineus, a medicinal plant known for its various therapeutic properties. The main active constituent of Starpril is Rosmarinic Acid, which has been identified as the primary compound responsible for its beneficial effects.

-
Product Phosphatidylserine
Phosphatidylserine powder (PS) is from the phospholipids family, which is the only one that could control the functioning of key membrane proteins. Usually it refers to a series of compounds of PS,in that the lipid-acetyl residues differs greatly in variety of product sources. Phosphatidylserine exists in ...
-
Product Red clover Extract
Red Clover Extract are isoflavones, which are important for the prevention of breast cancer, prostate cancer, colon cancer,osteoporosis and improving menopausal symptoms in women compared with other phytoestrogens. Therefore, Red Clover Flower Extract is widely used in health food. It is almost no heat, wi...
-
Product Ginger Extract
Gingerols are extracted from ginger root (zingiber officinale). Gingerols include 6-gingerol, 8-gingerol, 6-shogaol and 10-gingerol. The extract is brown yellow to light yellow powder, soluble in organic solvents, such as methanol, ethanol and DMSO.
-
Product Human Fibrinogen Concentrate
During blood coagulation, fibrinogen (coagulation factor I) is converted into fibrin by the serine protease thrombin (coagulation factor IIa). Fibrinogen has various applications in pharmaceutical and biotechnological manufacturing. It can be used as a coating on medical devices, and in wound healing. ...
-
Product POWDER SAMPLER
VALIANT Pharma Samplers are designe all kind of Samplers and Compaction Machine.
-
Product Two Function Closure
Dispensing closure.Just unscrew and release Your active ingredient into the bottle.
-
Product ilon Lip Cream HS
ilon Lip Cream HS is using the properties of patented micro algae active Spiralin® ingredient for care and protection before, during and after a lip herpes reactivation. The conclusion of a publication in the leading medical 'Journal of Allergy and Clinical Immunology' (JACI, 2016/01) from the results...
-
Product BioPhorum Sustainability
Our mission: To act as a voice of the industry to enable delivery of the industry’s sustainability ambition quickly and effectively. To lead innovative teams who have the capability and credibility to create consensus and ultimately drive real change that benefits people and planet.
Why col...
-
Product BioPhorum Supply Chain to Patient
Our mission: To transform the performance of global pharmaceutical clinical and commercial outbound supply chains through industry collaboration.
Why collaborate? The pharmaceutical outbound supply chain is facing capacity constraints more challenging than ever before. Increasing costs and g...
Biopharmaceuticals
Biopharmaceuticals, also known as biologic medical products, are drug products manufactured using biotechnology. Biopharmaceutical drugs are structurally similar to various compounds within the body. Over time, drug development has progressed from small-molecule compounds to large molecule proteins and other accompanying biopharmaceuticals. The discovery of biopharmaceuticals has significantly improved the healthcare delivery. It has provided a shift in providing cures for chronic illnesses such as refractory cancers, diabetes, autoimmune diseases, etc., thereby improving the affected patients' quality of life.
The use of biopharmaceuticals for therapeutic purposes dates as far back as the 19th century, with recombinant human insulin being the first approved biopharmaceutical used for therapeutic purposes in human beings. Over the last three decades, the biopharmaceutical industry has experienced notable growth and is expected to contribute to some remarkable pharmaceutical industry developments. Biopharmaceutical offers a wide range of advantages and benefits to pharmaceutics, and here, we would be exploring these benefits. We would also examine the biopharmaceutical manufacturing process, notable examples of biopharmaceuticals, and more.
What are biopharmaceuticals?
These complex medicines are proteins (monoclonal antibody), sugars (glycan), nucleic acids (RNA, DNA), living cells, or tissues extracted or semi synthesized from a biologic source by biotechnology for in vivo diagnosis or therapeutic purposes.
The majority of biopharmaceuticals are synthesized or derived from living organisms or genetically modified cells and organisms. They cannot only lessen symptoms or treat chronic diseases but also cure them with little or no side effects because of their origin and specificity. Some biopharmaceuticals include enzymes, antisense drugs, cytokines, monoclonal antibodies, gene therapy, cell therapy, etc.
Biopharmaceuticals have helped personalize the treatment process to meet the specific health needs of patients. This is achieved through the modification of drugs genetically through DNA-recombinant technologies or cell fusion to alter specific factors to cure, prevent, or diagnose individual diseases.
How are biopharmaceuticals made, and why?
As opposed to chemical synthesis through which conventional medicines and drug products are made, biopharmaceutical drugs are synthesized through more complex processes from living cells. With the rapid growth of the biopharmaceutical industry and the discovery of new biologic therapies, the term biopharmaceutical (biologics) refers to a variety of medicines and drug products. The diversified portfolio contains various drugs produced by different biotechnological methods and techniques.
These drug products are not made from chemical mixtures but rather from living organisms. Experts in cellular biology, biochemistry, and several other fields carry out biologic testing and analysis (using analytical methods) to ensure that the desired end product is made.
For example, biologics used to treat joint pain are made by adding a DNA into a living cell such as a yeast or mammalian cell to enable the production of a specific molecule in large amounts. These molecules are usually therapeutic proteins that are isolated and utilized as active ingredients.
When newly introduced, biopharmaceuticals focused solely on developing recombinant protein such as enzymes (TPA) and hormones (insulin, erythropoietin, growth hormone, etc.) through recombinant DNA techniques. With time, they have delved into the design and development of chemically defined cells, manufacturing of monoclonal antibodies, and genome-based technology. These new techniques have improved the pharmaceutical manufacturing process and created new treatment options for different diseases.
Like every other drug, biopharmaceutical drugs are produced for therapeutic purposes, but with a catch. They are produced majorly to cure chronic and dilapidating diseases such as cancer, diabetes, rheumatoid arthritis, and several others. The manufacturing of these drug products also aims at providing effective, safe, and cost-effective drugs for therapeutic purposes.
What is the biopharmaceutical industry?
The biopharmaceutical industry is an essential subset of the pharmaceutical industry. It is comprised of biopharmaceutical companies that discover, develop, produce, and market biopharmaceuticals. The drug products made are used to diagnose, treat, prevent, and cure diseases when administered to patients. This industry deals majorly in biopharma drugs and medicines produced via the biotechnological process from living entities such as micro-organisms, living cells, tissues, or organs of humans and animals. The biopharmaceutical industry successfully merges between biotech and pharmaceutical companies to provide better and safer healthcare to patients.
What is the difference between biopharmaceutic and biopharmaceuticals?
Biopharmaceutics is simply pharmaceutics that deals with biopharmaceuticals. It is a branch of pharmaceutical science that examines the physiochemical properties of drugs, their toxicology, pharmacology, dosage forms, and patients' clinical response to drugs, biopharmaceuticals inclusive. The recommended dosage and dosing interval varies for different drugs, and it significantly affects their efficacy and safety. Biopharmaceutics provide adequate information and understanding of the pharmacodynamics and pharmacokinetics of biopharmaceutical drugs, their metabolites, and their effects on the body. Through biopharmaceutics, biopharmaceutical drugs can be evaluated based on their properties and effects on the body.
On the other hand, biopharmaceuticals refer to inherently biologic pharmaceuticals extracted, synthesized, or semi-synthesized from live forms using biotechnological methods. They constitute a significant part of the drug products examined and evaluated in biopharmaceutics to improve healthcare delivery and, in turn, the quality of patients' lives.
How are biopharmaceuticals manufactured?
The rapid advancement of genetics and molecular biology has played a significant role in the biopharmaceutical manufacturing process. The introduction of new and refined techniques such as protein purification, host system, etc., has positively impacted the efficacy, capacity, and safety of biopharmaceuticals produced.
There are many biopharmaceutical manufacturing processes, and they vary based on the type of drug product to be produced.
Where is biopharmaceutical manufacturing heading?
With several emerging technologies in the biopharmaceutical manufacturing process, the biopharmaceutical manufacturing process is expected to take a huge turn. With over 1000 biopharmaceuticals in clinical trials, the majority of drugs in the future may be biopharmaceuticals. The increased need for biopharmaceuticals and the constant advancement in biotech will impact biopharmaceutical manufacturing. Given the various advantages of gene therapy, biopharmaceuticals' manufacturing process is likely to focus more on gene therapy in the future.
What is the process involved in the process of manufacturing biopharmaceuticals?
The process of small-scale and large-scale biopharmaceutical production varies. However, biopharma companies produce to indulge in large-scale biopharmaceutical production. The processes involved in manufacturing these drugs are complex, lengthy, and largely differ depending on the end-product. Generally, the processes include biosynthesis, purification, formulation, and final dosage preparations.
Materials and equipment are sterilized at the biosynthetic stage, and a cell culture or biologic extract is harvested or collected in a bioreactor to grow and multiply. During the purification process, cells, tissues, enzymes, or desired products are separated from surrounding nutrients and byproducts to produce the bulk product. Unlike conventional drugs mixed with other chemical components during formulation, biopharmaceuticals are made and sold as sterile products. Although they are sold as sterile products, biopharmaceuticals are packaged in similar ways as conventional drugs before distribution and sales.
This description gives a general knowledge of the manufacturing process carried out. Nevertheless, there are special intricate biotech processes that can be carried out and some include continuous manufacturing, and other processes requiring single use technology.
What special considerations must be made when manufacturing biopharmaceuticals?
Consistent safety, effectiveness, and quality are the essential factors that must be considered during the biopharmaceutical manufacturing process.
The manufacturing processes of each biopharmaceutical product must be designed to produce consistent quality and safe biopharmaceutical drug products. This involves the complete removal of contaminants and impurities such as viral contamination, endotoxins, cell membranes, and others from the separated product. Also, regular rigorous and well-documented checks and examinations on the efficiency and performance of the materials and equipment must be carried out to validate the manufacturing process.
Benefits of biopharmaceuticals
Given the structural composition and origin of biopharmaceuticals, these bio drugs provide several health benefits to patients and, in extension, the healthcare industry and healthcare professionals. The emergence of biopharmaceuticals has provided patients with a better shot at quality life and longevity.
How do patients benefit from biopharmaceuticals?
Biopharmaceutical drugs, unlike conventional medicines, mimic the structures of human compounds, and this gives biopharmaceuticals the potential and ability to cure diseases more effectively. They also produce fewer side effects due to their high specificity, unlike the traditional medications that are capable of affecting multiple systems simultaneously. Given their similarities with human compounds, they are very safe and highly effective.
Also, these drugs can be genetically modified to allow healthcare professionals to tailor treatments to meet patients' specific health needs. In addition, some generic drugs play crucial roles in providing patients with curative and diagnostic benefits.
However, one of the greatest potentials of biopharmaceuticals that also serves as an essential benefit to patients is gene therapy. Through gene therapy, conditions characterized by rapidly dividing cells due to mutated or defective genes, such as cancer, can be cured. Unlike some cancer drugs and other medications also, biopharmaceutical drugs are non-carcinogenic. The innovation of biopharmaceuticals also aids in producing varieties of vaccine to prevent infection and the spread of infectious diseases in patients.
Generally, bio drugs improve longevity, increase life expectancy, provide welfare to the population, and provides several public health benefits.
How does the healthcare industry benefit from biopharmaceuticals?
The impact of biopharmaceuticals and biopharmaceutical innovation on health care professionals also tells on the healthcare industry. Biopharmaceuticals have a massive positive economic impact, and other healthcare industry benefits that cannot be ignored. The development and availability of the various biopharmaceuticals in the pharmaceutical market will increase access to bio drugs, cost-effective medicines, and a boost in the global pharmaceutical market. Also, the ease of commercial production of biopharmaceuticals boosts the healthcare industry's economy and market growth.
How do healthcare professionals benefit from biopharmaceuticals?
Biopharmaceuticals and biopharmaceutical innovations provide outstanding benefits to healthcare professionals. It provides healthcare professionals with a variety of safe and efficient curative and therapeutic options for their patients. Therefore, in cases where a particular bio drug doesn't produce the desired therapeutic effect in a specific patient or has undesired side effects, other alternatives within the same drug family can be administered. Also, biopharmaceutical innovation provides job opportunities for healthcare professionals and individuals in different fields of practice.
Examples of common biopharmaceuticals
There are various biopharmaceutical products, and the majority of these products are widely known. Some common biopharma products include insulin, blood factor IX and VIII, human growth hormone, erythropoietin, tissue plasminogen activator, therapeutic antibodies, glucocerebrosidase, interferon α, and β, and GSCF. These form the most common examples, but they fall into the different biopharmaceutical classes, including hormones, clotting factors, blood products, vaccines, cytokines, and growth factors.
Where can some common biopharmaceuticals be found?
Biopharmaceuticals are derived and produced from various cells. They can be extracted and synthesized mammalian cell lines, microbial cells (yeast culture or recombinant E. coli), moss plants, and plant cell cultures. The majority of these sources are readily available and accessible at all times. These sources are origins from which the drug development process spans.
Who commonly uses biopharmaceuticals?
Biopharmaceuticals are primarily used for patients. However, most of the patient population that uses biopharmaceuticals are patients with long-term diseases such as psoriasis, diabetes, Crohn’s disease, rheumatoid arthritis, etc. The elderly constitute the majority of patients affected by either of these chronic conditions. Also, cancer patients form a significant percentage of the patient population using biopharmaceutical products.
What industries can common biopharmaceuticals be found in?
The common biopharmaceuticals are produced majorly by the biopharmaceutical and biotechnology industries. Within these industries, these biopharmaceutical products are developed and manufactured by biopharmaceutical companies. Some of the top pharma companies that manufacture the most common biopharma products include Gilead Sciences, Amgen, Genentech, Biogen, Riotech, Samsung biologics, Teva Pharmaceuticals, Celgene, Pfizer, United Therapeutics Corporation, UCB, and Sanofi. Although these companies form the existing biopharmaceutical manufacturing companies, a new biopharmaceutical company, Clover biopharmaceuticals, presently undergoing clinical trial is set to become a top tier pharmaceutical company in this industry.
References
https://medcraveonline.com/OAJS/biopharmaceutical-innovation-benefits-and-challenges.html
References
Upcoming Events
-
CPHI North America 2024
Pennsylvania Convention Center, Philadelphia
07 May 2024 - 09 May 2024 -
-
CPHI South East Asia 2024
Queen Sirikit National Convention Center, Bangkok, Thailand
10 Jul 2024 - 12 Jul 2024
Pharmaceutical Industry Webinars
-
Webinar Exploring Three Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
-
Webinar The Next Frontier – Emerging Opportunities in the LATAM Pharma Market
-
21st November 2023
-
4pm CET / 10am EST
-
-
Webinar Vistamaxx™ MED - imagine the possibilities for healthcare product performance
-
10th October, 2023
-
4pm CET / 10am EST
-
-
Webinar Co-processing: A Multifaceted Approach for Enhancing Density & Powder Flow
-
19th September 2023
-
4pm CET / 10am EST
-
-
Webinar The Outlook for Cell & Gene Therapy Manufacturing
-
28th June 2023
-
4pm CET / 10am EST
-
-
Webinar Contract Packaging Outlook: Growth Trends within the Commercial Packaging Sector
-
25th May 2023
-
4pm CET / 10am EST
-
-
Webinar The HPAPI Boom: How Pharma is Rising to the Manufacturing Challenge
-
23rd February 2023
-
4pm CET / 10am EST
-
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





























































































































.png)
.png)
.png)

.png)
.png)
.png)
.png)




.png)
.png)





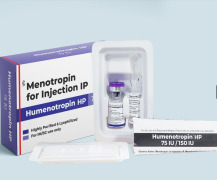
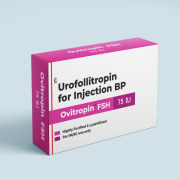







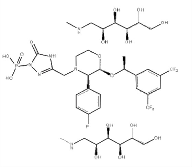



















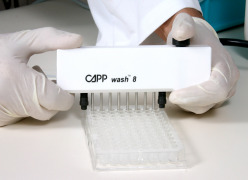





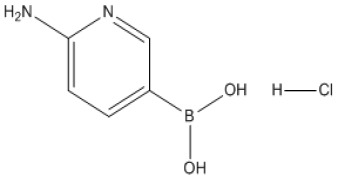
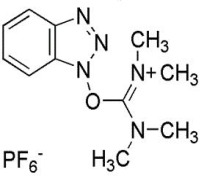







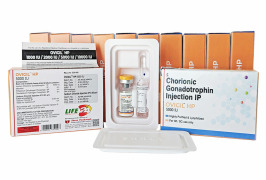



.png)

.png)




















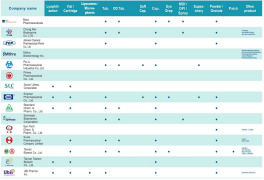








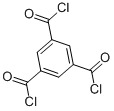
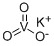
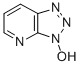
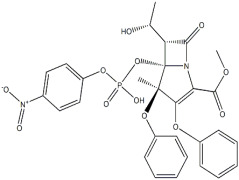






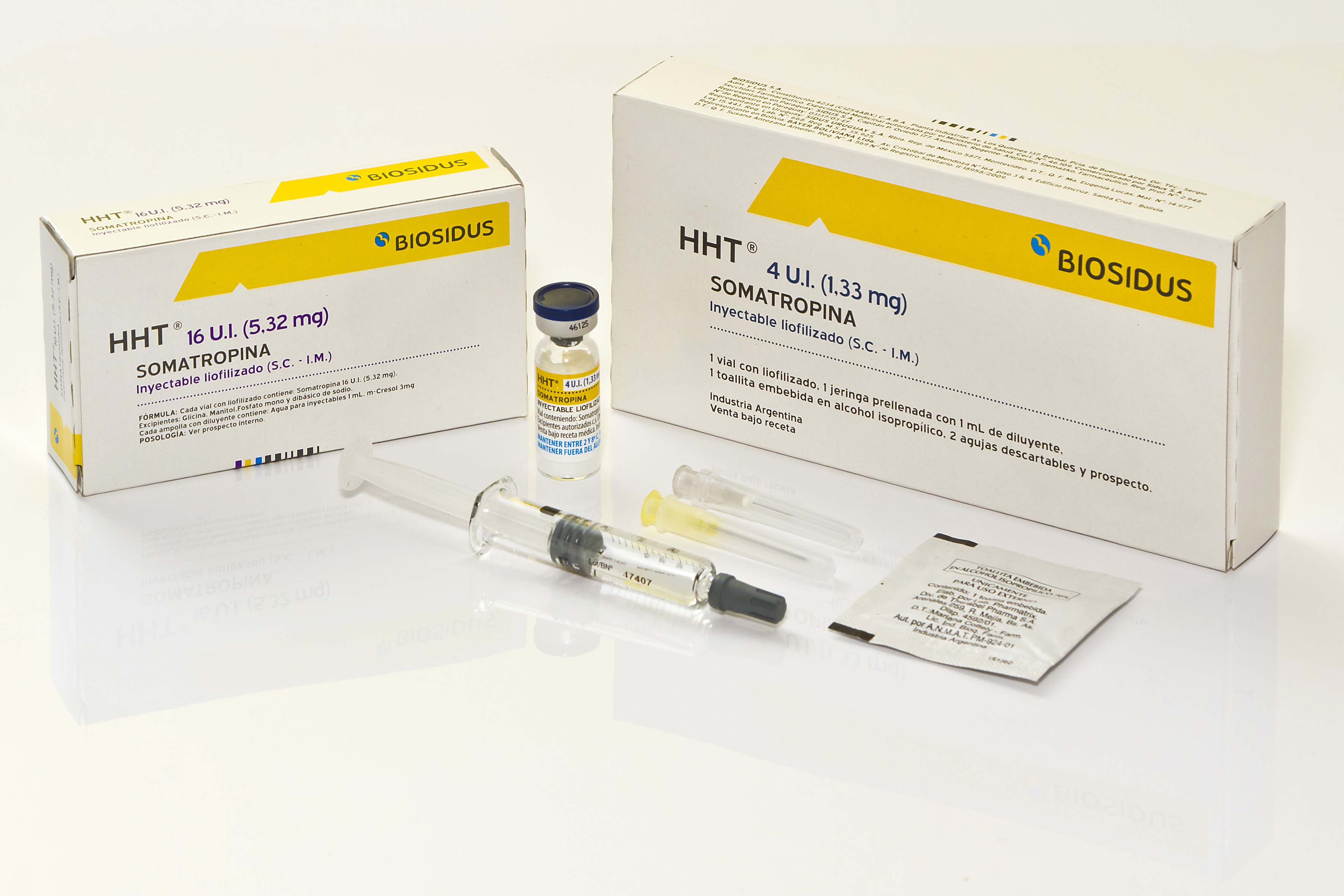
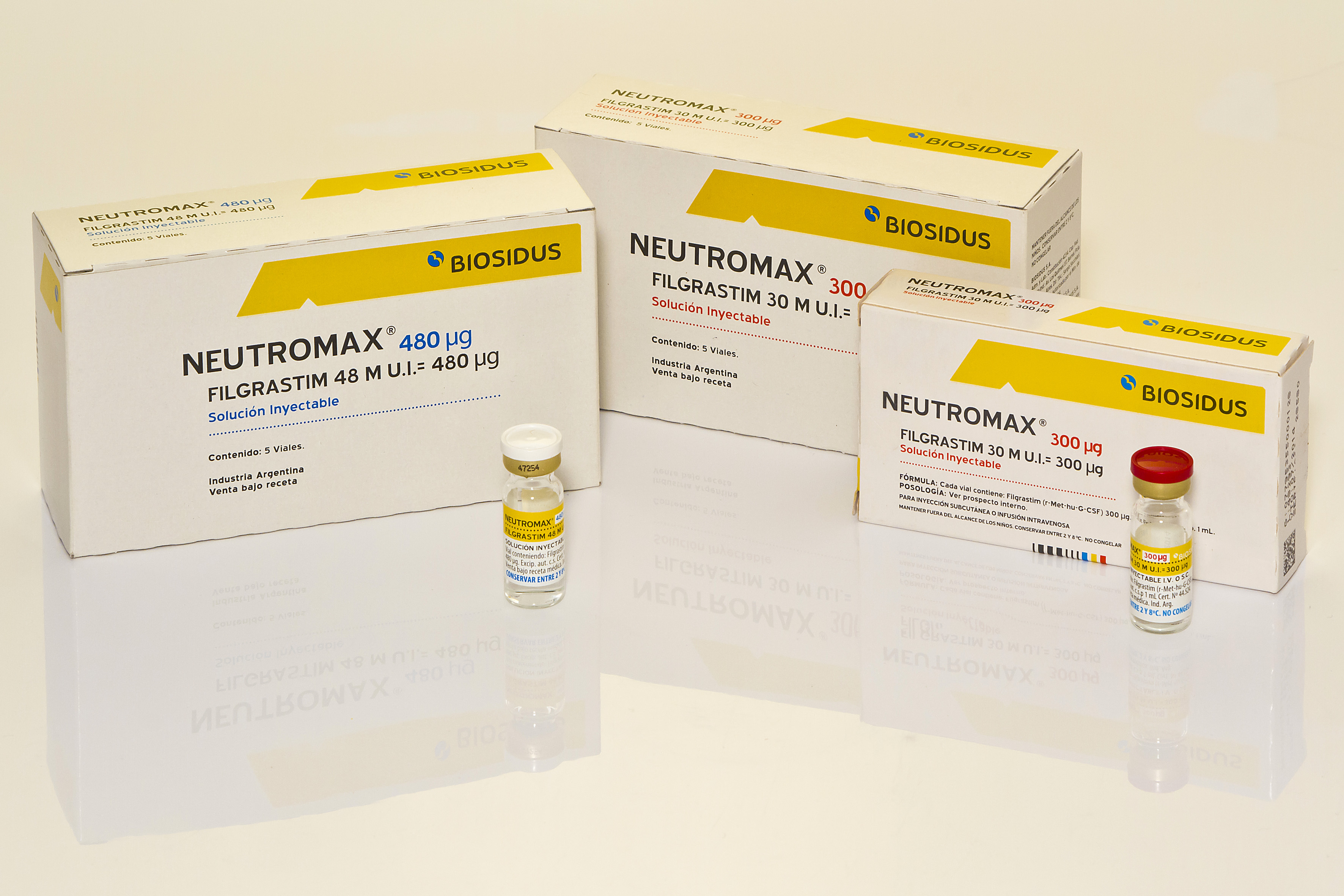
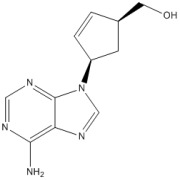
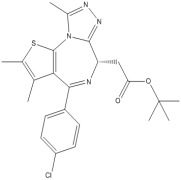
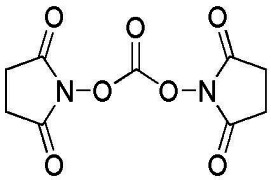
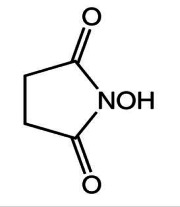




















.png)


.png)



.jpg)