Analytical Chemistry
Analytical Chemistry Companies (39)
Analytical Chemistry News
-
News Sai Life Sciences to boost biology capabilities at its integrated R&D campus
The CRDMO will expand in vitro and in vivo biology services, DMPK and toxicology capabilities. -
News Evotec expands into gene therapy with new Austrian unit
Evotec has established a dedicated gene therapy-based R&D site in Orth an der Donau, Austria with the immediate focus of spearheading a partnership with Japan’s Takeda to support its research projects, the German drug discovery firm said Monday. -
News GVK Bio continues to see growth tailwinds for 2020 thanks to a ‘crescendo of factors’
China-US trade challenges has led to de-risking by pharma companies. -
News ChengDu DaShengGe Pharmaceutical at CPHI China 2019
CPHI China 2019 World Pharmaceutical Raw Materials China Exhibition was held in Shanghai, China on June 18-20, 2016.
Analytical Chemistry Products (52)
-
Product Analytical Research and Development Services
Services for analytical research and development: As your organization focuses on core business goals, you'll require a partner who has a history of providing consistently high-quality regulated compliance testing. Our team are focused on supporting product development, GMP manufacturing and distributio...
-
Product Biopharmaceutical Analysis
Solvias has a solid reputation with market-leading biopharmaceutical companies for the characterization and QC analysis of monoclonal antibodies (mAbs), glycoproteins, PEGylated proteins and peptides and biosimilars (follow-on biologics).. This means that businesses of all sizes, even smaller biotechs, can...
-
Product Methanol Gradient HPLC Grade
Largest synthetic acetonitrile and high purity solvents manufacturer. • 35,000 t/a synthetic acetonitrile ; • 25,000 t/a high purity acetonitrile ; • 10,000 t/a HPLC solvents ; • 10,000 t/a solvent blending and packaging capacity.
-
Product Mikromol custom reference standards
Creating pharmaceutical reference standards to customer specification has always been at the heart of the Mikromol business. We understand that you are constantly discovering new actives and impurities of interest - each year, our dedicated, highly-qualified customs team produces several hundred new materi...
-
Product Method Development
We are specialist in the method development of analytical methods for analysis of API, Final Drug Products or low level impurities. Usually followed by full validation in accordance to ICH guidelines.
-
Product Fourier PAT
We celebrate the arrival of our synTQ by Optimal NMR adapter – opening the door to a new world of PAT for the Bruker Fourier 80. This brings a wealth of structural information and direct quantification to online chemical and bioprocess monitoring, resulting in further reductions in risk and cost.
-
Product Sodium Hyaluronate Sterile Grade
Sodium hyaluronate of high molecular weight could form a breathable film at the surface of skin to moisturize the skin, protect the skin from the bacteria , dusts and UV rays. While sodium hyaluronate of small molecular weight could permeate the derma, increase the blood circulation, improve the intermedia...
-
Product Analytical Services
The complete integration of analytical sciences into the process of API development is fundamentally understood at CARBOGEN AMCIS. Our scientists, technique ranges, systems (including a fully validated LIMS) and procedures facilitate a full understanding of the unique characteristics of the complex molecul...
-
Product Early Stage Development - Integrated Programs
Accelerating molecules through to proof-of-concept - Simplify early development and accelerate to Proof-of Concept.Proof-of-concept (POC) is a key milestone in the development of a new drug candidate. We understand the transition to POC needs to be fast and cost-effective.With fully integrated capabil...
-
Product Analytical Chemistry & Microbiology Services
Our expert team provides full analytical, microbiology and stability services to ensure that your raw materials, bulk product and finished products are qualified, analyzed and released to cGMP standards.
Our services include:
Analytical
• Raw material and drug product...
-
Product Nitrosamine Impurities Testing
SGS Life Sciences has considerable expertise in the method development of nitrosamine determination in pharmaceutical products. SGS has established a specific method for NDMA which can be applied to various different matrices. Alternatively, a platform method, based on trace-level detection by LC-MSMS, is ...
-
Product Quality Control
We offer a highly professional quality management throughout the organization. In more than 500 m² of laboratory facilities, we annually test approx. 1,500 product Batches . The area of responsibility for quality control already starts with choosing the appropriate suppliers. Hence, in...
-
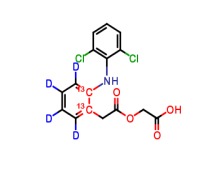
Product Aceclofenac-d4,13C2 (major)
Labelled Aceclofenac. Aceclofenac is an anti-inflammatory and analgesic drug.
-
Product Cannabidiphorol
Rare cannabinoids:CBC Series/CBG Series/CBD Series(CBC/CBCV/CBG/CBGP/CBDV/CBDB/CBDH/CBDP/CBDC8
-
Product Automated Total Nitrosamine Analyser
• Reaching low detection limits is essential for accurate nitrosamine analysis. The Automated Total Nitrosamine Analyser is sensitive enough to detect down to 1 ppb of NDMA. • The Automated Total Nitrosamine Analyser can analyse up to 10 samples per hour. Using this as a tool to screen samples for ...
-
Product Walk in Cooling Cabinet.
The Walk-In Cooling Cabinets / cold rooms are designed according to ICH guidelines, WHO, MCA and USFDA requirements to maintain uniform conditions. They are superior in airflow distribution, temperature control technology, cabinet construction and are manufactured as per cGMP regulations.
Biggest Walk...
-
Product Drug Stability Testing and ICH Storage Outsourcing
Testing of pharmaceutical stability and ICH storage outsourcing: Our extensive capabilities cover all ICH conditions (or custom conditions), including climate control walk-in chambers, cabinets, refrigerated and freezer storage that are fully monitored and controlled, with backup units at every locatio...
-
Product Analytical Services
Solvias provides cGMP-compliant contract analytical services to help you ultimately provide safer products to consumers. We provide a comprehensive range of analytical services to the pharmaceutical, biotech, medical devices and cosmetics industry with similar regulatory requirements for raw materia...
-
Product Extractables and Leachables Testing
Solvias provides expert testing and consulting services for Extractables & Leachables in single-use process materials and container closure systems. With our proprietary EXLEA database, we provide unique automated high throughput screening of more than 6000 Extractablesusing Accurate Mass LC/MS/MS Tech...
Upcoming Events
-
CPHI Korea 2024
COEX, Seoul, Korea
27 Aug 2024 - 29 Aug 2024 -
CPHI Milan 2024
Fiera Milano, Italy
08 Oct 2024 - 10 Oct 2024 -
CPHI & PMEC India 2024
India Expo Centre, Greater Noida, Delhi NCR
26 Nov 2024 - 28 Nov 2024
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
















-comp320105.png)































.jpg)














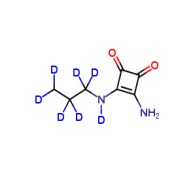
![9-(4’-Aminophenyl)-9H-pyrido[3,4-b]indole-d4](https://www.cphi-online.com/46/product/107/87/93/p0th_S.jpg)
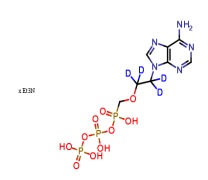
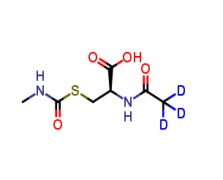









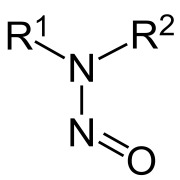

















.png)

.png)



.jpg)