Biomanufacturing
Biomanufacturing Companies (45)
Biomanufacturing News
-
News CPHI Milan Speaker Spotlight: Pharma Manufacturing and Localisation in Africa
In the run-up to CPHI Milan, we sit down with some of the experts and thought-leaders speaking at this year’s conferences. -
News Sanofi invests billions into Frankfurt insulin production site
French pharmaceutical company Sanofi have announced an investment of EUR1.3 billion at their existing BioCampus site in Frankfurt am Main for the expansion of insulin production. -
News What can you expect from CPHI Milan 2024?
CPHI Milan 2024 marks the 35th anniversary of CPHI. The 35th Edition of the show, taking place 8–10 October, promises to be a celebration of everything pharma, covering every sector, and featuring thought leaders and trailblazers shaking up the i... -
News New Aurigene biologics facility opens in Hyderabad, India
Aurigene Pharmaceutical Services Ltd. have opened a biologics facility in Hyderabad, India in a biocluster known as Genome Valley.
Biomanufacturing Products (64)
-
Product Biologic drug product CMO services - Fill and Finish
GC Biopharma is one of the top-tier biopharmaceutical companies in South Korea with over 55 years of history and advanced technology. GC Biopharma provides premium Fill & Finish service to clinical and commercial customers for vaccines, recombinant and biosimilar pharmaceutical products. We have state-of-t...
-
Product Biosimilars Portfolio
• Pegfilgrastim (Filed with EMA) • Filgrastim (Filed with EMA) • Trastuzumab (Filed with EMA, MHRA, Received recommendation for marketing authorization in India) • Bevacizumab (Filed with MHRA) • Omalizumab (Global PhIII ongoing) • Denosumab (Global PhIII ongoing) • Ranibizumab (Global PhIII ongoing...
-
Product Drug Substance Manufacturing - Mammalian Cell Cultures
• Around 430,000 L total commercial capacity for cell culture • Manufacturing sites around the globe: Biberach/Germany, Vienna/Austria, Fremont, CA/USA and Shanghai/ China- from 2,000 up to 15,000 L bioreactors • GMP facilities licensed by EMA, FDA, MHLW and other authorities ...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
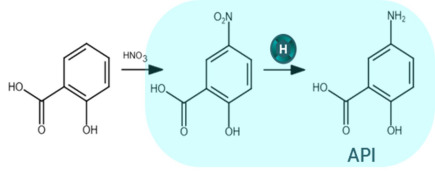
Product Semaglutide API
Complies with regulatory requirements: We can produce high-purity and high-quality standard raw materials that meet GMP requirements. They can be used for scientific research and also exported as raw materials for formulation. For more information, please consult us.
Complete quality documents:...
-
Product Technology Transfer
Maximize the production capabilities through a de-risking process with our comprehensive technology transfer services for engineering batches, process performance qualification (PPQ) batches, and commercial batches of vaccine antigens, including viral vectors, protein subunits, and virus-like par...
-
Product ULC Series 328
FARRAR® pioneered forced air convection cooling to answer the challenge of preserving and storing biological and human tissue donor samples and materials. Purpose-built for life science applications, the Ultra-Low Chamber ULC-190, ULC-259, and ULC-328 are the only -20°C to-80°C, forced air, ultra-low-t...
-
Product Sodium Hydroxide Solution
Sodium hydroxide compendial grade pellets blended with USP purified water. Can be packaged in drums or totes.
-
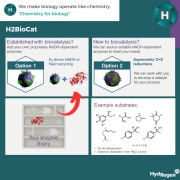
Product H2BioCat: asymmetric reductions
H2BioCat is an enzyme on carbon biocatalyst system for highly selective chemical synthesis via hydrogen-driven cofactor supply to any NADH/Flavin dependent redox enzyme
• 100% atom efficient cofactor recycling: mitigates waste from typical carbon based reductants for biocatalysis (glucose / alcohol) ...
-
Product Prionex®
Prionex® is a 10% aqueous solution of highly purified dermal collagen from porcine origin, known for its excellent protein-stabilizing properties. It is partially hydrolyzed, terminally sterilized, and free from cartilage, bone, and plasma components.
Key Advantages:
- Opti...
-
Product Supply Chain Management
• GMP Compliant Storage of a wide range of client products from API and excipients to consumables as well as both Stability and Retention sample programs. • Facility and Staff certified for storage, handling, and shipment of hazardous materials. Including Flammable, Toxic, Potent, etc. products. • Envir...
-
Product Pharmaceutical and Biological Products Contract Development and Manufacturing
Bora Pharmaceuticals is a premier international contract development and manufacturing organization (CDMO). Our six state-of-the-art cGMP manufacturing facilities across Asia and North America deliver to more than 100 markets around the world. Our sites have the highest industry standards for quality, dev...
-
Product Media & Feeds for CHO cell line
The First CHOice® Medium is a high-performance cell culture medium optimized for mammalian suspension cells ensuring high product quality and cell viability during all cultivation phases. In combination with the First CHOice® Feeds Alpha & Beta, offer a platform to achieve excellent cell growth, an...
-
Product PDRN (Polydeoxyribonucleotide)
PDRN (polydeoxyribonucleotide) is a mixture of deoxyribonucleotides derived from a controlled purification process of Salmon Trout or Chum Salmon sperm DNA which makes sure its safety and stability. PDRN activates the adenosine A2A receptors and generates nucleotides that can contribute to DNA formation, t...
-
Product WHEATON Roller Apparatus by DWK
WHEATON® roller apparatus are used for roller bottle cell culture, an established cell culture method and relatively inexpensive way to set up a flexible and scaleable biopharmaceutical operation. In addition to the standard product DWK also offer a wide range of customized options to accommodate the most ...
-
Product Fermentation
Relying on an experience gained over more than 50 years, OLON represent one of the most extensive know how of microbial fermentation in Europe. The Group, global leader in biomanufacturing, has two Biotechnology Centres located in Italy and is one of the first companies in Italy producing via microbial ...
-
Product Plasmid DNA
Manufacturing of plasmid DNA as starting material for:
• Clinical manufacturing of gene and immuno-oncology therapies, for example in AAV or lentiviral vector.
• Clinical manufacturing of biological drugs.
Quality Highlights:
...
-
Product Cell Expansion & Purification Applications (Upstream & Downstream)
PAN-Biotech empowers biotechnology by offering comprehensive solutions for both upstream and downstream applications in cell expansion and purification.
Upstream Applications:
- Cell Expansion: Our specialized media and supplements are designed to optimize cell growth ...
-
Product Microbial Services
We are a world-leader in process development and cGMP manufacturing of proteins derived from a range of microbial hosts including E. coli, P. pastoris and S. cerevisiae .
Upcoming Events
-
CPHI & PMEC India 2024
India Expo Centre, Greater Noida, Delhi NCR
26 Nov 2024 - 28 Nov 2024 -
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance









































.png)

.png)
.png)
.png)
.png)
.png)

.png)












.jpg)









































.png)






















.png)







.png)

.png)



.jpg)




