Bio Analytical Services
Bio Analytical Services Companies (20)
Bio Analytical Services News
-
News Sartorius extends its cell culture media business via acquisition
Acquiring Xell AG will enable Sartorius to offer specialised media for manufacturing viral vectors -
News Sartorius and RoosterBio partner to advance scale-up of hMSC manufacturing
The collaboration aims to accelerate the development and commercialization of groundbreaking cell-based regenerative cures -
News Sai Life Sciences to boost biology capabilities at its integrated R&D campus
The CRDMO will expand in vitro and in vivo biology services, DMPK and toxicology capabilities. -
News Stevanato Group to open US Technology Excellence Center in Boston
Due to begin operations in late September, the US TEC will bring value-added laboratory services to the heart of the biotech community.
Bio Analytical Services Products (41)
-
Product Biosimilars Testing Services
Testing services for biosimilars: Our biosimilar analytical comparability programs provide highly relevant data for early stage characterisation and later stage comparison. These programs evaluate and compare all pertinent features of the biosimilar product and are based on the ICH Q6B. Programs encompa...
-
Product Analytical Services (GMP and R&D)
Our analytical portfolio comprises 200+ different techniques – all performed by Coriolis’ experts in-house. Our offering includes:
• Aggregate analytics • Particle characterization • Particle identification • Surfactant characterization • Higher order structure analysis • Funcional assays • Chemi...
-
Product Bioanalytical Services
Scorpius BioManufacturing develops custom assays to profile your molecule and test your clinical trial samples, allowing the seamless progression of your product throughout its lifecycle. Beginning with the end in mind, we design pre-clinical analytical test methods and IND-enabling programs that easily ...
-
Product Bioequivalence tests for topical drugs
We are a GLP lab specialist in vitro permeation testing, including the bioequivalence tests under the EMA draft guideline. We support pharmaceutical companies through bioequivalence studies. Visit our booth and find out more about our R&D dedicated to this topic.
-
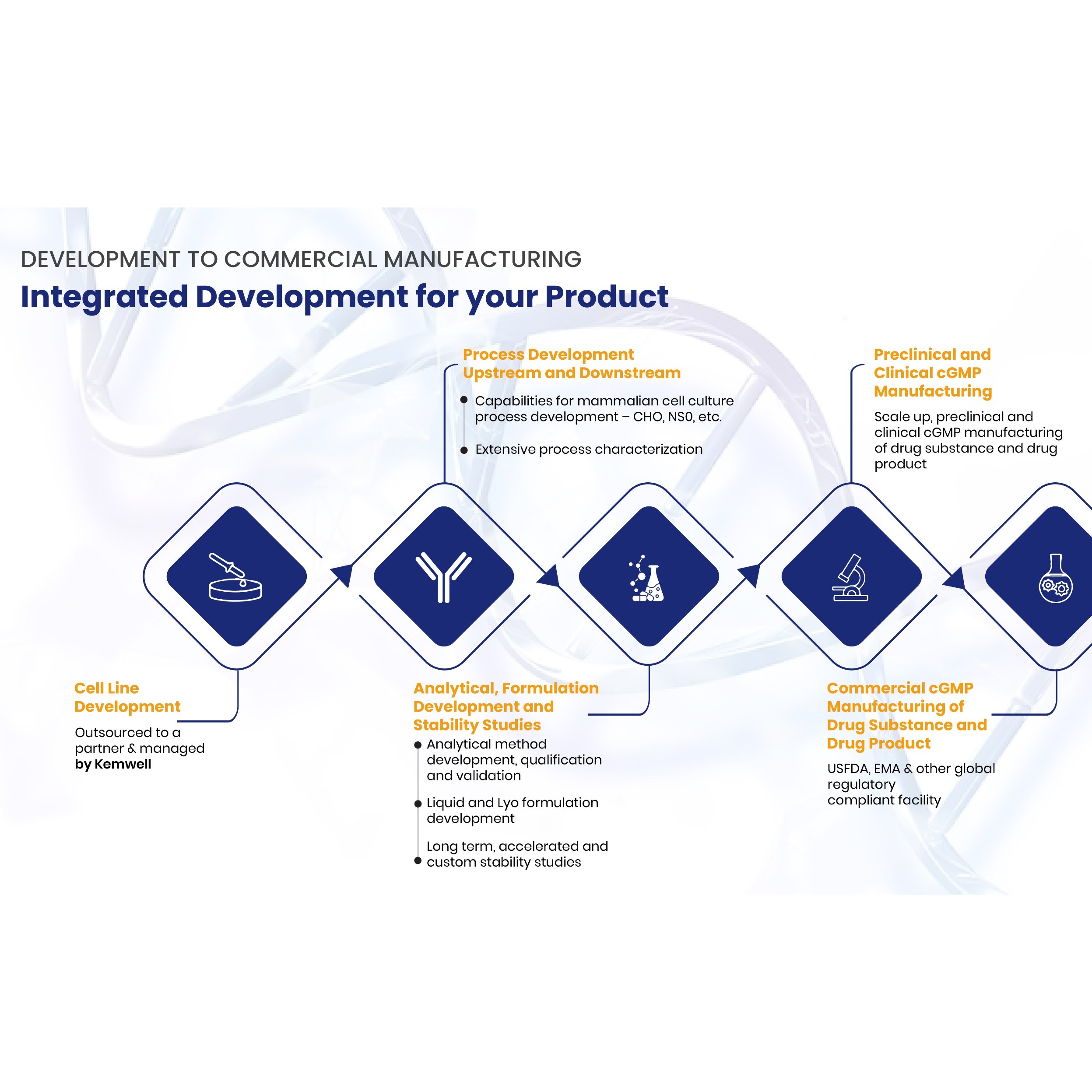
Product Kemwell Biopharma
SERVICES Kemwell provides integrated development and manufacturing services for companies that require one-stop solution for mammalian cell-culture based protein therapeutics The team is experienced to undertake end-to-end activities right from cell line development till cGMP clinical and commercial manufa...
-
Product Method Development
We are specialist in the method development of analytical methods for analysis of API, Final Drug Products or low level impurities. Usually followed by full validation in accordance to ICH guidelines.
-
Product Bioanalytical Services
To effectively support your drug development project and maximize your R&D productivity, NUVISAN offers a wide array of bioanalytical solutions for small and large molecules. Starting at the discovery, all the way through phase IV, we offer expertise bioassays and high-sensitivity ligand-binding assays...
-
Product Clinical Solutions: Bioanalysis of small and large molecules
We provide a wide range of GCP/GLP bioanalytical services for preclinical and clinical studies with human and/or veterinary drug products up to Phase III. We offer development and validation of bioanalytical methods in different biological matrices; complete bioequivalence studies; pharmacokinetics/toxicok...
-
Product Analytical Services
Solvias provides cGMP-compliant contract analytical services to help you ultimately provide safer products to consumers. We provide a comprehensive range of analytical services to the pharmaceutical, biotech, medical devices and cosmetics industry with similar regulatory requirements for raw materia...
-
Product Cell Based Assays
Kymos Pharma Services, S.L. offers wide range of bionalysis services in medicinal chemistry which includes cell based assays. Expertise in cell biology enables us to implement, validate and perform cell-based assays (cba) for several applications like potency assays or neutralizing antibodies. Kymos work w...
-
Product Analytical Services
Mabion offers a extensive suite of Analytical Services to ensure safety, efficacy and quality of your biologic product. Our portfolio includes development, optimization and validation of analytical methods, as well as stability studies, GMP release testing and in-depth drug characterization. In addition to...
-
Product Bioanalysis of Biologics & Biosimilars
At Selvita, we offer comprehensive bioanalytical services for a wide range of biological drugs, including innovative proteins, biosimilars, therapeutic peptides and enzymes, biomarkers, monoclonal antibodies, hormones, nucleic acids, and other large molecules. As a GLP-certified and GCP-compliant multinati...
-
Product Simon Caplan
Samedan and PM Group are a Pharmaceutical and Healthcare publishing house. We will be exhibiting the following magazines: 1. European Biopharmaceutical Review(EBR) 2. Pharmaceutical Manufacturer and Packing Sourcer (PMPS) 3. International Clinical Trials (ICT) 4. INnovations in Pharmaceutical Technology(IP...
-
Product Microbiological Analysis
Oasis Test House caters to the microbiological requirements for its clients serving them with the below analysis. • Preservative Efficacy Analysis • Sterility Test • Sterility Validation • Microbial Limit Test Validation • Microbial count and pathogens • Bioburden testing • Antibiotics assay • Vitam...
-
Product Consulting & Project Management Services
BIOCOM AG offers wide range of services which includes consulting & project management services. It has gathered a huge amount of knowledge and gained a wealth of experience in all areas of biotechnology, from pharmaceuticals and medical technology to the agricultural sector and industrial production. Base...
-
Product Bioequivalence studies
Intertek offers wide range of pharmaceutical services which includes bioequivalence studies. It belongs to bioanalytical services for preclinical and clinical studies category. Contact us for more information.
-
Product Cell-based Neutralization Assays
Experienced cell-based neutralizing antibody assay capabilities. Our experts, at our GLP/GCP compliant laboratory, evaluate each therapeutic, conduct tailored assay development and ensure that each assay is optimized to suitably determine the presence of NAb with maximum possible level of drug toleran...
-
Product Bioanalytical Services (GLP/GCP)
Services for Bioanalysis (GLP/GCP): In our state-of-the-art facilities, we provide GLP/GCP method development, validation, method transfer, sample analysis and pharmacokinetic and toxicokinetic support, along with automated data collection and reporting systems. Over the past 20 years, we have supported...
-
Product GCP/GLP Bioanalysis Services
In our state-of-the-art facilities, we provide GLP/GCP method development, validation, method transfer, sample analysis and pharmacokinetic and toxicokinetic support, along with automated data collection and reporting systems. Over the past 20 years, we have supported the development of pharmaceuticals, bi...
-
Product Comparability Studies for Biopharmaceuticals
We design bespoke analytical programmes that ensure that the relevant quality attributes for your drug substance or product are evaluated to support your manufacturing process changes. We select analytics referenced by the ICH Q6B to examine the product's structural features (primary, secondary and&nb...
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance























.jpg)
.jpg)
.jpg)
.jpg)

.jpg)
































.png)







.png)

.png)



.jpg)


