C2 PHARMA’S Upcoming Launch of Oxybuprocaine Hydrochloride
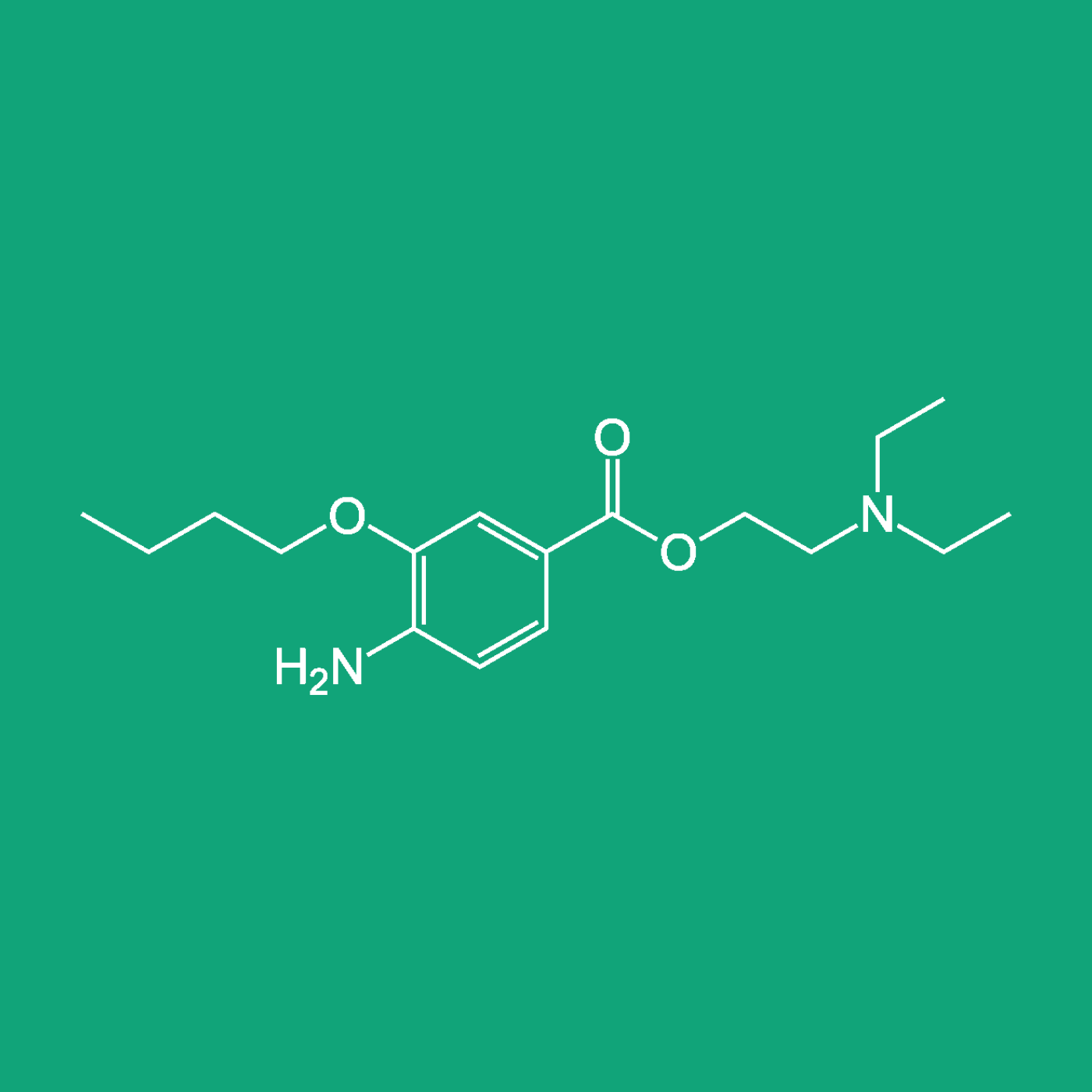
C2 Pharma is excited to announce the upcoming launch of their new Ophthalmic API, Oxybuprocaine Hydrochloride. To learn more check out the full press release.
C2 PHARMA, the global leader in ophthalmic and niche active pharmaceutical ingredients (APIs), is excited to announce the upcoming launch of their new API, Oxybuprocaine HCL, an API that will be manufactured by our manufacturing partner Laurus Labs. We are completing the validation process, by April 2024, and upon completion, GMP samples will be available to our customers worldwide for evaluation and testing.
We expect to submit a Certificate of Suitability (CEP) by May 2024, a US-Drug Master File (US-DMF) by August 2024, and a Certificate of Acceptance of Drugs and Food (CADIFA) in Q4 of 2024.
The addition of this API will extend our range of high-quality Ophthalmic APIs and strengthen our commitment to meeting the market's evolving needs.

Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance