Document Management System
.png)
Product Description
AmpleLogic

-
IN
-
2018On CPHI since
Categories
Specifications
AmpleLogic

-
IN
-
2018On CPHI since
More Products from AmpleLogic (8)
-
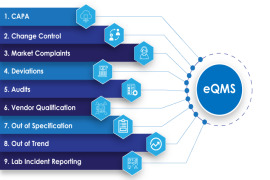
Product AmpleLogic Quality Management System Software
AmpleLogic QMS Software automates quality processes like CAPA, Change Control, Deviation, OOS, OOT, Audit Management, Vendor Qualification in pharma and life-science manufacturing companies who do it manually. All the AmpleLogic modules are 21 CFR Part 11 and EU annexure 11 compliant. Automating QMS helps ... -
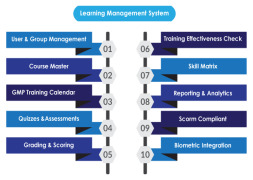
Product Learning Management System
AmpleLogic GMP Learning Management software is built to manage pharma specific employee training and Training life cycle starting from Induction till employee’s total tenure in the organisation as per 21 CFR Part 11, EU ANNEX, GMP and other regulatory requirements. AmpleLogic Biochemical Learnin... -
Product Stability Schedules Management and Tracking Software:
Stability Schedule Calendar helps to keep track of different schedules of the testing with reminders and escalations.Details like Stability Protocol reference number, Stability Protocol Version etc. can be maintained.Separate flow for each Stability Protocol, Stability Initiation Protocol and Incubatio... -
Product HPLC (High-Performance Liquid Chromatography) Column Usage Tracking Software
The major problem faced by pharmaceutical companies and labs in column usage is maintaining the continuous updates on cumulative injections and duplicate mapping of the same column for different products at the same time. AmpleLogic HPLC Column Management offers users to create an inventory of their HPLC c... -
Product Calibration Schedules Management and Tracking Software
AmpleLogic calibration management software directs calibration cycles and preventive maintenance schedules for all equipment’s. It helps in scheduling and recording the results of all your calibration activities to maintain certifications in compliance with several quality and regulatory standards. Our ... -
Product Annual Product Quality Review (APQR)
Annual Product Quality Review (APQR) will be conducted for each commercial product manufactured in the Pharmaceutical, Biotech and Bio-similar Industry. The aim of this annual product review within the pharmaceutical companies is to verify the consistency of the process, to assess trend, to determine the n... -
Product Electronic Log Book
AmpleLogic Electronic Log Book is a web-based application specifically deals with the requirements related to the Calibration, Equipment Usage, Cleaning, Stability Schedule, Standards Usage, Chemical Usage Logs, and other details log for all equipment’s. This eLog book application conve... -
Product Low Code Application Development Platform
AmpleLogic’s Low Code application development Platform is process management tool & comprises robust workflow that connects every person, department and systems inside and outside of your business.No Code application development platform allows to create any number of business applications on our own. ...
AmpleLogic resources (1)
-
Brochure AmpleLogic GMP Compliance Software for Quality Operations
The brochure explains in brief about AmpleLogic, list of products, list of customers, implementation methodology followed and why AmpleLogic companies should choose AmpleLogic for their Quality Process Automation.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance