Computer software
Computer software Companies (22)
Computer software News
-
News Sanofi to collaborate with OpenAI and Formation Bio
Pharmaceutical giant Sanofi have announced a collaboration with Formation Bio and OpenAI to build AI-powered software for accelerated drug development. -
News Using AI to determine drug compounds to curb the opioid crisis
Researchers have developed a computer model, that learns using AI, to identify potential drug compounds that can be used to block opioid receptors in the brain. -
News N-SIDE launches Production App to optimise clinical manufacturing process
The software consulting company claims its software solution has already saved one pharma company €10.5 million -
News Blockchain could galvanize COVID-19 impacted healthcare supply chains: GlobalData
Blockchain technology could prove to be the missing link for global healthcare supply chains exposed by the COVID-19 pandemic in building resilience and galvanizing efficiency, transparency and authenticity, according to market research firm, GlobalDat...
Computer software Products (55)
-
Product Pharmacovigilance - PvEdge - AI Enabled Drug Safety Database Suite
AI-enabled, cloud-ready,360 pharmacovigilance solution for safety database management. An end-to-end globally compliant safety software for Drugs, Devices, Vaccines and Combinational products from case intake to submissions and reporting. • Case Intake • Case Processing • Case Submission • Risk Manage...
-
Product IT and OT Infrastructure Qualification
Global Verification & Validation is a SaaS solution for Life Science companies that complies with EMA/FDA. FIVE Validation developed a SaaS platform that allows validation studies to proceed 6x faster than a manual (electronic or paper-based) process.

-
Product OC11 Word Platform
The OC 11 Platform is not just another software; it's the next generation of Global GxP and Data Integrity solutions. It's designed to revolutionize how GxP records are generated and to ensure users comply with global GxP and Data Integrity regulatory requirements..
Convert any Word form i...
-
Product AmpleLogic Quality Management System Software
AmpleLogic QMS Software automates quality processes like CAPA, Change Control, Deviation, OOS, OOT, Audit Management, Vendor Qualification in pharma and life-science manufacturing companies who do it manually. All the AmpleLogic modules are 21 CFR Part 11 and EU annexure 11 compliant. Automating QMS helps ...
-
Product Quality Management System
MasterControl Inc. is the most trusted provider of cloud-based quality and compliance software for life sciences and other regulated industries. Our mission is to help our customers bring life-changing products to more people sooner. MasterControl Quality Excellence is our flagship quality management syste...
-
Product Update Yourself with Latest version of L4-L5 Solutions and Russia Regulation - Presentations at PMEC Booth
Please click below Link to Register Now
Link :- https://forms.gle/N3jwpeShVq9GB4Xy9
-
Product CSDB 3.0
The serialisation solution must integrate production lines, partners, customers & suppliers - this means comprehensive integration, adaptation of processes and high security measures. Whether at the intersections between ERP software and production and packaging machines, to the systems of ex...
-
Product SoftGroup® SaTT Site Controller
SoftGroup® SaTT Site Controller is a centralized serialization software system for the management of the serialization and aggregation processes. The Site management software by SoftGroup provides a complete solution for managing the whole production of the factory and preparing serialization and aggregati...
-
Product LAB ICONICS ELN (ELECTRONIC LAB NOTEBOOK)
Lab Iconics ELN designed to manage the whole laboratory experiments, projects, forms and templates, and effectively manage the digital track of inventories.
-
Product Regulatory - KnowledgeNET - RIMS | eCTD | eCSV
KnowledgeNET is a global dossier publishing & management software empowering pharma industries to achieve regulatory compliance efficiently.
• eCTD Publishing: DMF | ASMF | CEP | ANDA | IND | BLA | PADER | PSUR | 510(K) • CTD/ ACTD and Regional Submissions • Query and Life Cycle Management ...
-
Product Manufacturing - ProcessXE - Smart Manufacturing & Automations Suite
Sarjen Systems' ProcessXE is a SMART manufacturing solution tailored for the pharmaceutical industry. This solution provides the following offerings
• Weighing & Dispensing Management • Electronic Batch Manufacturing Record • Electronic Batch Packaging Record • Equipment Qualification...
-
Product Quality - QEdge - Enterprise Quality Management Suite
Sarjen Systems’ QEdge is an enterprise-wide Quality Management System (QMS) specifically designed for pharmaceutical manufacturing. This risk-based QMS offers a comprehensive suite of solutions, including:
• Process Control • Document Control & Issuance • Training Management System • Ve...
-
Product AI and non-AI validation technologies for Life Science companies
We specialize in IA and non-AI Computer System Validation, equipment validation, utilities qualification, and IT/OT infrastructure qualification. Our services comply with the regulatory standards of the EMA, FDA, WHO, and other key Life Science authorities across Europe and the Americas. With a proven trac...
-
Product GO!FIVE Digital Validation Solution
Global Verification & Validation is a SaaS solution for Life Science companies that complies with EMA/FDA. FIVE Validation developed a SaaS platform that allows AI and traditional validation studies to proceed 6x faster than a manual (electronic or paper-based) process.
-
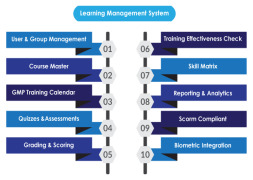
Product Learning Management System
AmpleLogic GMP Learning Management software is built to manage pharma specific employee training and Training life cycle starting from Induction till employee’s total tenure in the organisation as per 21 CFR Part 11, EU ANNEX, GMP and other regulatory requirements. AmpleLogic Biochemical Learnin...
-
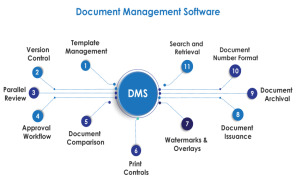
Product Document Management System
Challenges like lack of proper document escalation flow, improper document revision and getting document signatures made AmpleLogic to design and develop Electronic document management system. In Pharmaceutical companies each and every activity within a GMP regulated environment such as US FDA, MHRA ...
-
Product Manufacturing Execution System
MasterControl Inc. provides cloud-based software to empower manufacturers in life sciences and other regulated industries to digitize and streamline their processes to reduce errors on the shop floor. Our mission is to help our customers bring life-changing products to more people sooner. Manufacturing Exc...
-
Product Track and Trace Software
With SoftGroup® SaTT Communication Cloud you can securely communicate across your business network. With fully automated communication or with XML file exchange through the sFTP service, we can help you to enhance your supply chain visibility by keeping your costs to a minimum and reducing human errors.
-
Product Serialization and Aggregation Software
SoftGroup® SaTT Line Controller is serialization software installed on a serialization machine, which controls the process of printing and verifying the process of the printed templates with and without serialization for each batch.
-
Product Regulatory Compliance Software
SoftGroup® SaTT Gateway is the most user-friendly and fully automated SaaS software for meeting compliance for traceability and serialization. It fully supports the EU FMD, Asl belgisi, and MDLP requirements as well as other implemented and emerging compliance regulations for each member of the supply chai...
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




















.png)
.jpg)
.jpg)
























































.png)







.png)

.png)



.jpg)


