Haselmeier GmbH
About Haselmeier GmbH
Certifications
Categories

-
DE
-
2021On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
Meet us at
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025
Products from Haselmeier GmbH (5)
-
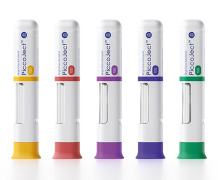
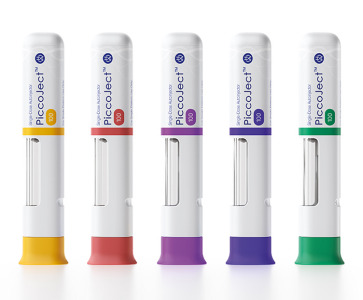
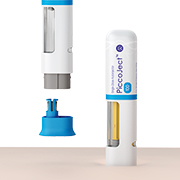
Product PiccoJect Autoinjector
PiccoJect is a highly compact, customizable and fully featured two-step autoinjector designed for subcutaneous delivery of drug products.
• Extremely low part count: Only 8 parts in total – reduces manufacturing and scale up challenges • Ergonomic shape & small size: Thanks to its compact flat f... -
Product D-Flex Injection Pen
D-Flex, the pen platform for subcutaneous self-injection. Bridging the gap between fixed and variable-dose pens, this innovative product also paves the way for connected commercial devices.
• Just one pen for many needs: Disposable pen for fixed or multiple fixed dosages • The versatile solution: Allo... -
Product Re-Vario™ & Re-Vario™ A Injection Pens
The Re-Vario is a re-usable pen platform with a anodized aluminium body to provide years of reliability. The Re-Vario™ A is a high quality, plastic re-usable pen.
The product platform (Re-Vario™ / Re-Vario™ A) is the basis for a re-usable, variable dose injection device designed for use... -
Product In-Pen Reconstitution
With one approved product and several more in development, Haselmeier has experience with multiple solutions that allow easy and convenient administration of freeze-dried biologics.
In-Pen Reconstitution with a Reusable Pen In this solution, each drug cartrdige is supplied with a disposable cartridge ... -
Product PiccoJectâ„¢ Autoinjector
PiccoJectâ„¢ is a highly compact, customizable and fully featured two-step autoinjector designed for subcutaneous delivery of drug products.
Extremely low part count: Only 8 parts in total “ reduces manufacturing and scale up challengesErgonomic shape & small size: Thanks to its compact flat form t...
Haselmeier GmbH Resources (4)
-
Webinar Case Study: Importance of Simulation and Modeling in Autoinjector Development
In this webinar, originally broadcast as part of Pharmapack Europe 2022, Chris Muenzer, Vice President of Innovation & Development, Haselmeier, a medmix Brand discusses how development of an integrated drug delivery device is a complex exercise that requires expertise in medical device engineering and pharmaceutical science. Health authorities expect legal manufactures to have a strategy to control device performance through the entire supply. Modeling and simulation is an important tool to ensure robust and repeatable performance while maintaining time to market. This case study looks at how Haselmeier is applying these techniques during the creation of a new autoinjector platform. In addition, Chris will look at future opportunities for further improvements though expanded use of digital twins and other technologies. -
Video PiccoJect Video
PiccoJect™ is a highly compact, customizable and fully featured two-step autoinjector designed for subcutaneous delivery of drug products.Extremely low part counrtLarge wrap-around drug window with customizable sizeOptimized spring forcesFuture proofCommitted to sustainabilityErgonomic shape & small sizeColored status indicatorAudible clicks at the start and end of injectionAny standard 1 ml or 2.25 ml pre-filled syringeA full-service platform
The products shown in this video are under development and some of them may not yet have been approved for sale under applicable medical device regulations. The concept provides general information about these products, their field of application and intended use, and their function, performance and specification are subject to customer specific development and may deviate from those shown herein. All information contained herein is directed at medical device manufacturers and pharmaceutical companies. This information shall not constitute a promotion for use in a way which conflicts with any applicable medical device regulations, nor is it directed at patients or intended to replace professional medical advice. -
Webinar Sustainable Drug Delivery: The Device Supplier's Perspective
This session will explore: The role of the device supplier in overall product sustainability Benefits of lifecycle assessment (LCA) in device development Trade-offs between usability, cost of goods, and carbon footprint Challenging Pharma to close the circle and enable better solutions -
Webinar Advanced Delivery Device Technology to Simplify the Reconstitution of Lyophilized Drugs
Lyophilization (freeze drying) is a proven process for increasing the shelf life of vaccines, biologics and other injectables. But delivering these products requires several use steps that make them challenging to administer, particularly by patients and caregivers in non-clinical settings. To address these needs, Haselmeier has been developing several solutions for an easier and more convenient reconstitution and administration. This presentation will cover different types of primary drug containers such as vials and dual chamber cartridges for reconstitution along with the available device solutions to simplify delivery. The challenges and advantages of each solution, including vial to cartridge, out-of-pen reconstitution, and in-pen reconstitution will be discussed.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance










-file145616.png)
