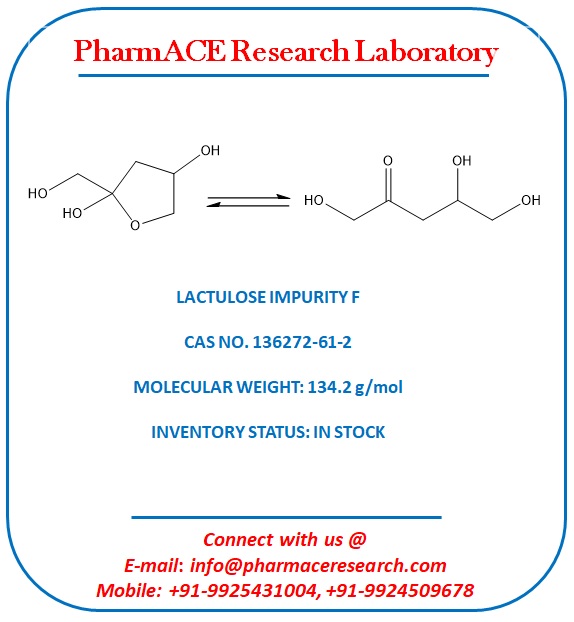
Lactulose Impurity F
Product Description
Pharmace Research Laboratory

-
IN
-
2022On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Primary activities
Categories
Specifications
Pharmace Research Laboratory

-
IN
-
2022On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Primary activities
More Products from Pharmace Research Laboratory (2)
-
Product Cefdinir Impurities Standards
Cefdinir Isoxazole ImpurityCefdinir Sulphoxide
Cefdinie Lactone
Cefdinir E-Isomer
Cefdinir 7-Epimer
N-Acetyl Cefdinir
O-Acetyl Cefdinir
Cefdinir Related Compound A
-
Product N-Nitroso Rasagiline
N-Nitroso Rasagiline - Impurity of Rasagiline
Pharmace Research Laboratory resources (1)
-
Brochure Pharmace Research Laboratory
Manufacturers of Reference standards, working standards, Impurity Standards, Metabolite Standards and Deuterated standards.Custom synthesis as per client’s needs. We have expertise in NCE development, Research Molecule synthesis and FTE based projects.
Library of more than 2000 standards and are expanding every day. Provide detailed COA with Mass, IR, HNMR, HPLC, CNMR, TGA.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance