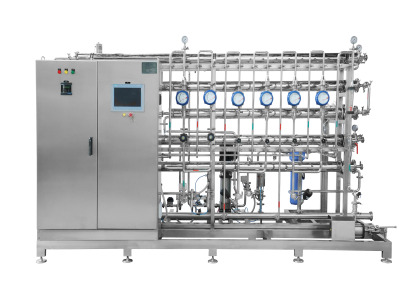
Liquid Oral Manufacturing Plant ( Syrup Manufacturing Plant)

Product Description
Sterinox Systems

-
IN
-
2017On CPHI since
-
25 - 49Employees
Company types
Primary activities
Categories
Specifications
Sterinox Systems

-
IN
-
2017On CPHI since
-
25 - 49Employees
Company types
Primary activities
More Products from Sterinox Systems (7)
-
Product Purified Water (PW) Treatment Plants (RO-EDI Plants)
Purified Water (PW) Treatment Plants (RO-EDI Plants) generates High Purity water for pharmaceutical, Biotech, Semiconductor and ‘Food & Beverages Industry.
Sterinox Purified Water Treatment plants are designed and optimised for hassle free low maintenance operation. The entire syste... -
Product Multi Column Distillation Plant ( WFI Generation Plant)
Multi Column Distillation plant ( Water for Injection Generation Plant) produces low conductivity pyrogen / endotoxin free Water For Injection (WFI) by stage distillation process. Our Plant is based on falling Film Evaporator Technology. • Capacity varies from 5 to 6,000 litres per hour • Desig... -
Product Pure Steam Generator
The Pure Steam Generator is used to produce Pyrogen/ Endotoxin free Sterile Steam. The Pure Steam Generator (PSG) utilizes a falling-film type evaporator which produces pyrogen free pure steam, using a unique separation technology. Feed water is continuously pumped to the top of the evaporator heat exc... -
Product PW Distribution SKID
• PW and WFI Storage Tanks are manufactured as per cGMP guideline s & ASME VIII, DIV 1 • standard Storage tanks can be plain, jacketed or limpet type, provided by suitable rockwool/glasswool insulation and AISI 304 stainles ssteel cladding. • If required, provision of elec... -
Product Point of Use Heat Exchanger (POU HE)
Our extensive engineering expertise, unique designs and manufacturing techniques provide the most compact heat exchangers offering high heat transfer area, low utility consumptions for thermal exchange application and quick delivery of the product.
Our POU HE (Point of Use Heat Exchanger)... -
Product CIP and SIP System
Cleaning-In-Place (CIP) and Sterilization-In-Place (SIP) are systems designed for automatic cleaning and disinfecting without major disassembly and assembly work. We design, develop, manufacture, supply and install Mobile and Fixed CIP & SIP Units for sanitization and sterilization. The units are custo... -
Product STERILE SOLUTION MANUFACTURING VESSEL
Sterile Solution Manufacturing vessels are required to manufacture liquid injectable products. We adhere to stringent manufacturing processes and follow cGMP / ASME BPE guidelines to manufacture the Sterile Vessels. Manufacturing and Holding Vessels are accompanied with suitable high grade filtration t...
Sterinox Systems resources (1)
-
Brochure Sterinox Brochure
We introduce our company M/s Sterinox Systems as one of the leading pharmaceutical machinery manufacturers in India. We are a technology driven quality conscious engineering organization.
We are into the designing & manufacturing of pharmaceutical machineries. We have a range of machineries and equipments for injectable, liquid & ointment sections. Given below is a list of machines, equipments / Plants being manufactured by us.
Also Please find attached our company brochure for furthermore information on our products.
By implementing benchmarking technologies in manufacturing process and setting up fully-equipped in house quality, we are committed to international standards, We at Sterinox, ensure the high Quality products for our customers. We manufacture as per cGMP (current Good Manufacturing Practice) and adhere to our quality standards stringently. We have a QAP (Qua...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance