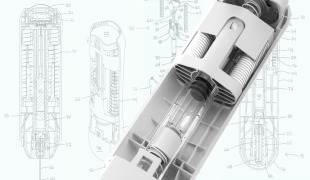
Medical Device Design & Engineering

Product Description
Duoject Medical Systems Inc

-
CA
-
2020On CPHI since
Categories
Duoject Medical Systems Inc

-
CA
-
2020On CPHI since
More Products from Duoject Medical Systems Inc (2)
-
Product Regulatory Support
Armed with decades of experience and a mature Quality System, we collaborate with you to ensure your medical device is market-ready and complies to all latest industry regulations.
Our capabilities and experience include:
- Fully integrated Quality System- Diversified & a... -
Product IP Creation
For every project Duoject works on, we strive to create a strong IP background to ensure our client’s commercial success for many years to come.
Duoject Medical Systems Inc resources (1)
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance