MicroBiopharm Japan Co., Ltd.
About MicroBiopharm Japan Co., Ltd.
Certifications
Categories

-
JP
-
2017On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Products from MicroBiopharm Japan Co., Ltd. (3)
-
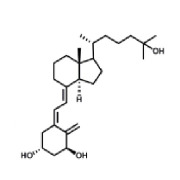
Product Calcitriol
MicroBiopharm Japan Co., Ltd. Offers a wide range of products which includes calcitriol. Please contact us for more information.Files : US-DMF, CEP, Japan-MF, China-DMF -
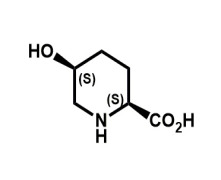
Product Cis-5-Hydroxy-L-Pipecolinic Acid
Spec; In-house
5-OH-L-(-)-Pipecolinic acid is a rare amino acid and used as an intermediate for β-lactamase inhibitors. We manufacture it by bioconversion from L-lysine and provide on industrial scale.
-
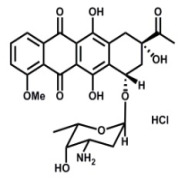
Product Daunorubicin Hydrochloride
MicroBiopharm Japan Co., Ltd. Offers a wide range of products which includes daunorubicin hydrochloride. Contact us for more information.Filed as starting materials of other anthracyclines.
MicroBiopharm Japan Co., Ltd. Resources (1)
-
Brochure MBJ_Brochure_E
English version
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance