Policy & Regulation News
Policy & Regulation news
-
News AbbVie's Mavyret receives FDA approval for the treatment of chronic hepatitis C
FDA approval is supported by an overall 98% cure rate in patients who received the recommended duration of treatment . -
News NICE approves Abraxane for pancreatic cancer
Life-extending pancreatic cancer medicine to be made available immediately on the NHS. -
News FDA grants Genentech’s Alecensa Priority Review
Designation given for initial treatment of people with ALK-positive lung cancer. -
News BMS receives FDA approval for Opdivo Injection for metastatic colorectal cancer
Approval based on CheckMate -142, in which Opdivo demonstrated an objective response rate of 28% among patients who received prior treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. -
News Amgen submits sBLA for Prolia in glucocorticoid-induced osteoporosis
Results from the Phase III study included in the sBLA submission showed that treatment with Prolia for 12 months led to statistically significant greater gains in BMD at the lumbar spine and total hip compared to risedronate. -
News BMS's Orencia receives second EC approval in less than a year
New approval for treatment of active psoriatic arthritis (PsA). -
News New research highlights lack of confidence in serialization preparations
40% of industry professionals admit they are not clear about the global requirements for serialization. -
News Merck launches Remicade biosimilar for all eligible indications, in the US
The biosimilar's list price will represent a 35% discount to the current list price of Remicade. -
News Novartis receives positive CHMP opinion for Rydapt
If approved, Rydapt would represent the first targeted treatment for newly diagnosed FLT3-mutated AML in the EU. -
News GSK receives FDA approval for a new self-injectable formulation of Benlysta
The approval marks the first subcutaneous self-injection treatment option for patients with systemic lupus erythematosus. -
News FDA approves new treatment to reduce the risk of breast cancer returning
Nerlynx is the first extended adjuvant therapy for patients with HER2-positive breast cancer. -
News FDA approves Impax's AB rated generic Concerta Extended-Release Tablets CII
Company expects to launch by the end of the year. -
News FDA committee recommends approval of Mylan and Biocon's proposed biosimilar trastuzumab
Vote marks first proposed biosimilar trastuzumab to be recommended by the committee. -
News Novartis CAR-T cell therapy CTL019 recommended for FDA approval
A BLA for this indication is under FDA priority review; if approved, CTL019 could become first CAR-T cell therapy available. -
News FDA accepts sNDA for Xeljanz for the treatment of adult patients with active ulcerative colitis
Anticipated Prescription Drug User Fee Act (PDUFA) action date for the sNDA is March 2018 . -
News Lilly reaches settlement agreement in US Cialis patent litigation
Cialis exclusivity is now expected to end at the earliest on 27 September 2018. -
News FDA approves Blincyto to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia
Blincyto is the first and only Bispecific T cell Engager (BiTE) immunotherapy to demonstrate superior overall survival versus standard of care chemotherapy. -
News SMC says ‘yes’ to Opdivo for blood cancer patients
Patients in Scotland will now join those in England and Wales and be able to benefit from this potentially life-extending immunotherapy on the NHS. -
News BMS's Orencia receives FDA approval for treatment of active psoriatic arthritis in adults
Orencia now approved in three autoimmune diseases. -
News UK Supreme Court rules in Lilly's favour on Alimta vitamin regimen patents
Actavis's products directly infringe Lilly's vitamin regimen patents in the UK, France, Italy and Spain. -
News Almac Discovery granted Orphan Drug Designation for ALM201 programme in ovarian cancer
ALM201 is a therapeutic peptide developed to mimic some of the properties of the naturally occurring protein FKBPL. -
News Impax launches additional strengths of generic Focalin XR extended-release capsules CII
Company has immediately initiated commercialization activities. -
News Novartis receives EU approval for Cosentyx label update
Label update includes 52 week data from CLEAR study demonstrating long-term superiority of Cosentyx versus Stelara in psoriasis. -
News Specialist CRO expands pharmacovigilance capabilities
Pharma clients now have direct and immediate access to a fully compliant safety database without needing to invest in their own system or use an interim solution to ensure compliance. -
News BIOCAD’s rituximab biosimilar to receive MA in India
First shipment of BIOCAD’s Acellbia to India is scheduled for September 2017. -
News Lilly's Olumiant gets the thumbs up in Japan for the treatment of rheumatoid arthritis
In clinical studies, baricitinib has demonstrated significant improvement in the signs and symptoms of RA compared to standard-of-care therapies. -
News FDA approves Vectibix for use in wild-type RAS metastatic colorectal cancer
First-and-only fully human monoclonal anti-epidermal growth factor receptor antibody approved by the FDA for this patient population. -
News Novartis receives EU approval for first-line use of Zykadia in ALK-positive advanced NSCLC
In ALK-positive advanced NSCLC patients, Zykadia demonstrated superior median progression-free survival compared with SOC chemotherapy with maintenance. -
News QS Pharma receives approval from EMA for commercial manufacture
The CDMO is now approved to manufacture medicinal products for the US, Europe and Japan. -
News Technology selection identified as biggest hurdle in serialization race for compliance
Resourcing the deployment of serialization systems and processes also cited as a significant barrier to compliance with European FMD, -
News Cladribine Tablets receives positive CHMP opinion for treatment of relapsing forms of MS
Cladribine Tablets is the first and only investigational medicinal product to have shown a sustained 4 years of disease control with a maximum of 20 days of oral treatment over 2 years in clinical trials. -
News Pfizer receives expanded Health Canada approval for Ibrance in HR+, HER2- metastatic breast cancer
New indication supported by results of Phase III PALOMA-3 Trial of Ibrance in combination with fulvestrant. -
News Biogen’s Imraldi, an adalimumab biosimilar candidate referencing Humira, granted positive opinion by CHMP
If approved, Imraldi will be the third anti-TNF biosimilar in Biogen’s portfolio in Europe. -
News FDA approves Mydayis – a once-daily option for ADHD symptom control in patients 13 years plus
Mydayis demonstrated improvements lasting up to 16 hours post-dose, beginning at 2 or 4 hours post-administration, compared with placebo, in total score on a skill-adjusted math test that measures attention in ADHD. -
News Novartis achieves regulatory milestone for AMG 334 in migraine prevention with EMA filing acceptance
AMG 334 (erenumab) is the first anti-CGRP monoclonal antibody developed for migraine prevention to receive EMA regulatory filing acceptance. -
News FDA accepts Amgen's sBLA to expand indication for Xgeva to include multiple myeloma patients
Xgeva is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumours and is the number one prescribed agent by oncologists for this indication in the US. -
News Sandoz receives approval in Europe for Rixathon to treat blood cancers and immunological diseases
Sandoz now has four biosimilars approved in Europe - more than any other company. -
News FDA updates on Pfizer drug shortages
Pfizer says shortages caused by manufacturing, distribution and third party delays. -
News FDA accepts Sandoz regulatory submission for a generic version of Advair Diskus
Sandoz believes its combination product will offer asthma and COPD patients same safety and efficacy as reference medicine. -
News Achelios Therapeutics concludes successful Type C meeting with FDA regarding Topofen
Currently there are no approved topical NSAIDs indicated for the treatment of migraine. -
News FDA requests removal of certain prescription opioid for risks related to abuse
Injection abuse of reformulated Opana ER has been associated with a serious outbreak of HIV and hepatit.is C -
News FDA Advisory Committee to review Avastin biosimilar candidate
The Committee will review analytical, pharmacokinetic and clinical data from studies involving ABP 215, including results from a Phase III study in patients with NSCLC. -
News Spinraza approved in the EU as first treatment for spinal muscular atrophy
Robust data from Phase III studies demonstrated positive impact on motor milestone achievement; increased survival in infants with SMA. -
News NICE approves first use of Opdivo in blood cancer
NICE has recommended immunotherapy drug nivolumab for the treatment of adult patients with classical Hodgkin lymphoma. -
News Sandoz proposed biosimilars adalimumab and infliximab accepted for regulatory review by the EMA
Biosimilar infliximab alone could potentially save the NHS £89 million. -
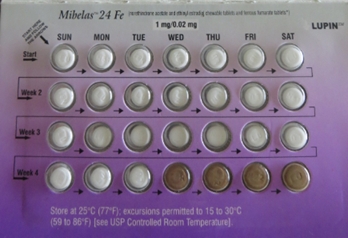
News Lupin Pharmaceuticals announces nationwide recall of Mibelas 24 Fe tablets
Recall due to out of sequence tablets and missing expiry/lot information. -
News FDA accepts for priority review BMS’s application for Opdivo in previously treated hepatocellular carcinoma
Application is based on results from the Phase I/II CheckMate -040 trial. -
News WuXi STA, CFDA and Jinshan Government to co-host Marketing Authorization Holder regulatory summit in Shanghai
Industry and regulators combine to run afternoon congress on the implications of regulatory changes. -
News FDA approves Kevzara for the treatment of moderately to severely active RA in adults
Kevzara is now available to US patients. -
News FDA approves Genentech’s Actemra for giant cell arteritis
Sixth FDA approval for Actemra since its US launch in 2010, -
News EMA validates application for BMS’s Sprycel in children with chronic myelogenous leukemia
Proposal extends application to the treatment of children and adolescents with chronic phase Philadelphia-chromosome positive chronic myelogenous leukemia and to the powder for oral suspension. -
News Saneca Pharma receives confirmation of multi-dosage cGMP approval for Russia
Certification covers the manufacture and packaging of hard and soft gel capsules, liquids for external use, semi-solids such as ointments and film coated tablets. -
News Aptar Pharma’s electronic Lockout device approved by EMA
The e-Lockout device being the first and only fully integrated electronic nasal drug delivery device to be approved by a US or European regulatory authority. -
News Research reveals manufacturers and contract packagers may miss serialization deadlines
Many companies currently unprepared because they lack the internal resources to devote to serialization. -
News Hikma receives CRL regarding its ANDA for generic Advair Diskus
Company believes low likelihood of approval this year. -
News Merck's Keytruda scores another FDA approval
FDA approves Keytruda as first-line combination therapy with pemetrexed and carboplatin for patients with metastatic nonsquamous non-small cell lung cancer (NSCLC) irrespective of PD-L1 expression. -
News First approval for Keytruda in a hematologic malignancy in the EU
European Commission approves Keytruda for patients with relapsed or refractory classical Hodgkin lymphoma who failed ASCT and BV or who are transplant-ineligible and have failed BV. -
News AstraZeneca’s Imfinzi receives FDA accelerated approval for previously treated patients with advanced bladder cancer
Imfinzi is the cornerstone in an extensive immuno-oncology program across multiple cancer types and stages of disease. -
News Impax announces FDA approval and launch of a generic version of Vytorin
One of the first companies to offer a generic version of Vytorin. -
News Takeda announces FDA Accelerated Approval of Alunbrig
Alunbrig approved for ALK+ metastatic non-small Cell lung cancer patients who have progressed on or are intolerant to crizotinib. -
News China FDA approves country’s first all-oral regimen for chronic hepatitis C, Daklinza in combination with Sunvepra
Daklinza and Sunvepra combination approved for genotype 1b, the most common chronic hepatitis C (HCV) genotype in China; combination has a 91-99% cure rate. -
News Shire granted EU conditional marketing authorisation for Natpar for the treatment of chronic hypoparathyroidism
Natpar is the first and only licensed recombinant human parathyroid hormone therapy for chronic hypoparathyroidism. -
News Regeneron and Sanofi receive FDA approval of a new once-monthly dosing option for Praluent Injection
Monthly dosing schedule now approved in both US and EU. -
News Recipharm delivers first serialised batch to Saudi Arabia
Company has invested €300,000 into the project, which is part of a wider €40 million investment into new serialisation technology and processes to comply with the European Falsified Medicines Directive. -
News Sandoz proposed biosimilars rituximab and etanercept recommended for approval in Europe
Company receives positive CHMP opinions for biosimilars rituximab and etanercept to treat immunological diseases. Biosimilar rituximab also recommended to treat blood cancers. -
News FDA approves Genentech’s Lucentis for diabetic retinopathy
First and only medicine FDA-approved to treat all forms of diabetic retinopathy. -
News FDA issues complete response letter for baricitinib
Additional clinical data are needed to determine the most appropriate doses. -
News FDA approves new indications for Harvoni and Sovaldi
First HCV direct-acting antivirals approved for use in adolescents. -
News Merck receives CRL from the FDA for TECOS study with sitagliptin
CRL relates to Merck's sNDA for Januvia, Janumet and Janumet XR. -
News Hikma enters into settlement agreement with Jazz for sodium oxybate
Agreement terms are "favourable for both parties". -
News Amgen submits applications in the US and Europe to expand current indication for Xgeva
Applications include data from the largest international trial conducted in multiple myeloma. -
News FDA now happy with Alkem's API facility
Company's CAPA addresses the three Form 483 observations. -
News Novartis drug combination Tafinlar + Mekinist receives EU approval for BRAF V600-positive advanced NSCLC
New indication of Tafinlar and Mekinist in advanced NSCLC provides only therapy approved in the EU for BRAF V600-positive NSCLC. -
News FDA approves Genentech’s Ocrevus for relapsing and primary progressive forms of MS
An important new treatment option for people with relapsing forms of multiple sclerosis demonstrating superior efficacy on the three major markers of disease activity compared with Rebif. -
News Tesaro announces FDA approval of Zejula for women with recurrent ovarian cancer
US commercial launch planned for late April. -
News Regeneron and Sanofi announce FDA approval of Dupixent
First targeted biologic therapy for adults with moderate-to-severe atopic dermatitis. -
News Pfizer receives positive CHMP opinion for Trumenba for prevention of meningococcal group B disease
Trumenba has been studied in a global clinical development program evaluating the vaccine in adolescents and adults. -
News BMS receives positive CHMP opinion recommending Opdivo for head and neck cancer
Opdivo already approved by the EC for six indications in four distinct tumour types. -
News FDA approves Parkinson's disease drug
First NCE approved for PD patients with motor fluctuations in the US in more than a decade . -
News Mylan receives tentative approval for "TLE400" under PEPFAR
TLE400 is a fixed-combination containing Efavirenz, Lamivudine and Tenofovir Disoproxil Fumarate Tablets, 400 mg/300 mg/300 mg. -
News FDA approves Novartis' Kisqali as first-line treatment for HR+/HER2- metastatic breast cancer
Kisqali plus letrozole showed treatment benefit across all patient subgroups regardless of disease burden or tumour location. -
News Shire receives European approval for label extension of Cinryze
Cinryze is the first and only Hereditary angioedema treatment approved for routine prevention in paediatrics. -
News Merck's Keytruda receives FDA approval to treat patients with refractory classical Hodgkin lymphoma
Only anti-PD-1 therapy approved for the treatment of patients with difficult-to-treat cHL regardless of prior stem cell transplantation or use of brentuximab vedotin -
News Colorado unapproved drug and dietary supplement makers ordered to cease operations for federal violations
Dietary supplement products "misbranded" and unapproved new drugs being marketed with drug claims despite not being approved for any use. -
News Mylan announces global settlement and license Agreements with Genentech and Roche on Herceptin
Settlement provides Mylan with global licenses for its trastuzumab product. -
News Boehringer's investigational anti-CD33 monoclonal antibody gains orphan drug designation for treatment of myelodysplastic syndromes
BI 836858 was previously granted orphan drug designation for the treatment of patients with AML. -
News FDA accepts sNDA for Vraylar
Application seeks to expand Vraylar label to include Phase III clinical data for the maintenance treatment of schizophrenia. -
News First ever biosimilar of interferon beta-1a approved in Russia
Represents Biocad's third authorized medicine for the treatment of relapsing-remitting multiple sclerosis. -
News NICE expands positive recommendation for Erbitux as first-line treatment for RAS wild-type mCRC
More patients now able to receive a standard-of-care treatment with Erbitux plus Folfiri or Folfox. -
News FDA accepts BLA for avelumab for the treatment of metastatic urothelial carcinoma for Priority Review
Second Biologics License Application accepted by the FDA for avelumab. -
News CHMP recommends EU Conditional Marketing Authorisation for Natpar for patients with chronic hypoparathyroidism
If approved, Natpar would be the first licensed recombinant parathyroid hormone in Europe for the management of chronic hypoparathyroidism, the only endocrine-deficiency disorder with no hormone treatment. -
News FDA expands approval of Spiriva Respimat inhalation spray
Steroid-free Spiriva Respimat now approved as asthma treatment for age 6 and older. -
News FDA accepts BLA for Mylan and Biocon's proposed biosimilar pegfilgrastim for review
The proposed biosimilar to Neulasta is used to reduce the duration of neutropenia and the incidence of fever associated with neutropenia in adult patients treated with chemotherapy in certain types of cancer. -
News Valeant's treatment for plaque psoriasis gets FDA thumbs up for Siliq
More than 50% of patients in three clinical studies who used Siliq achieved total skin clearance within a year. -
News EC approves once-daily Olumiant tablets for treatment of adults with active RA
Baricitinib, marketed as Olumiant, is the first JAK inhibitor approved to treat RA in the EU. -
News Pharma Explained: TEST
In the first video in our new series; Pharma Explained, Francess Zipp of Lachman Consultants explains why Good Manufacturing Practices (GMPs) are important and how a strong quality risk management system can ensure adherence to the GMPs. -
News Appeals Court grants stay of permanent injunction for Praluent during appeals process
Praluent continues to be available to patients in the US. -
News Lilly's Trulicity label updated to include use in combination with basal insulin for adults with type 2 diabetes
Trulicity is a once-weekly glucagon-like peptide-1 (GLP-1) receptor agonist injectable prescription medicine. -
News FDA approves first new treatment in more than a decade for secondary HPT in adults on hemodialysis
Intravenous administration puts delivery in hands of healthcare provider. -
News Amgen's Repatha significantly reduced the risk of cardiovascular events in FOURIER outcomes study
Landmark Repatha cardiovascular outcomes study meets primary and key secondary endpoint. -
News FDA accepts two sBLAs for Merck's Keytruda for metastatic urothelial cancer
Keytruda also receives Breakthrough Therapy Designation for second-line treatment. -
News FDA approves BMS's Opdivo for bladder cancer
Opdivo has now been approved in six tumour types in just over 2 years. -
News Merck gets the thumbs up in Europe for Keytruda to treat patients with metastatic NSCLC
First anti-PD-1 therapy approved in Europe for previously untreated patients with metastatic NSCLC. -
News EPO upholds decision regarding Sprycel
Patent found to be invalid. -
News Sanofi's Xyzal Allergy 24HR approved for OTC use in the US
Xyzal is an oral antihistamine with a proven 24-hour effect. -
News Novartis' Votubia receives EU approval to treat refractory partial-onset seizures in patients with TSC
Decision marks the third TSC-related indication for Votubia in the EU. -
News Mylan wins US District Court ruling related to Copaxone 40 mg/mL patents
Patents in question invalidated based on obviousness. -
News FDA approves Allergan's sNDA for Avycaz to include new Phase III data in patients with cUTI
New clinical data for Avycaz demonstrates efficacy in cUTI patients including those with infections due to resistant Gram-negative pathogens. -
News Sunovion’s Latuda receives FDA approval to treat adolescents with schizophrenia
First treatment approved in 5 years for adolescents aged 13-17 years with schizophrenia. -
News First adalimumab biosimilar candidate recommended for EMA approval
Amgen receives positive CHMP opinion for biosimilar adalimumab. -
News FDA approves 72 mcg dose of Linzess for adults with chronic idiopathic constipation
New dosage strength expected to be available in first quarter of 2017. -
News FDA approves IND for Vectura's VR647
VR647 is a drug/device combination, using the AKITA JET smart nebuliser, for the delivery of nebulised budesonide. -
News Endo agreement to resolve FTE investigation and litigation
No admission of wrongdoing, No monetary payment by Endo. -
News Concert Pharmaceuticals receives FDA orphan drug designation for CTP-656 for the treatment of CF
Topline results from the Phase II trial are expected by year-end 2017. -
News EMA validates Gilead’s MAA for investigational chronic Hep C therapy
Sofosbuvir/Velpatasvir/Voxilaprevir cranted an accelerated assessment by the EMA. -
News FDA approves Allergan's Rhofade cream, 1% for persistent facial erythema
Once-daily treatment reduces persistent facial erythema associated with rosacea through 12 hours. -
News Suliqua approved in the EU for the treatment of adults with type 2 diabetes
Suliqua will be delivered in two pre-filled SoloSTAR pens, providing different dosing options that may help answer individual market and patient insulin needs. -
News Tesaro receives CRL for rolapitant IV from FDA
No concerns raised by FDA related to the rolapitant IV efficacy or safety profile and additional clinical studies are not required. -
News New-generation mealtime insulin approved in Europe
Novo Nordisk to launch Fiasp in first half of 2017. -
News VivaGel BV granted QIDP and Fast Track designation by FDA
Product receives designations for both the BV treatment and prevention indications.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance