Vaccines
Vaccines Companies (21)
Vaccines News
-
News Bavarian Nordic narrows focus to meet demand for mpox vaccine in Africa
The WHO declares a state of emergency in Africa as mpox cases rise, and vaccine maker Bavarian Nordic works with institutions and government bodies to ensure equitable access to treatment. -
News Novartis and Viatris latest facing lawsuit over HeLa cell misuse
Global pharmaceutical companies Novartis and Viatris are the latest hit with a lawsuit claim pertaining to alleged misuse of the ‘HeLa’ cell line from the estate of woman whose cancerous tissue cells were taken without consent. -
News Tumour microproteins could provide basis for cancer vaccines
A recent study published in Science Advances has identified a set of microproteins produced exclusively in liver tumours, which may provide a clear target for the development of a cancer vaccine. -
News Pharma giants battle over COVID-19 patents in London courts
Two key players in the COVID-19 vaccine story – Pfizer/BioNTech and Moderna – have begun the latest chapter of their global legal battle over patents concerning technology for the development of mRNA therapeutics such as the COVID-19 vaccin...
Vaccines Products (52)
-
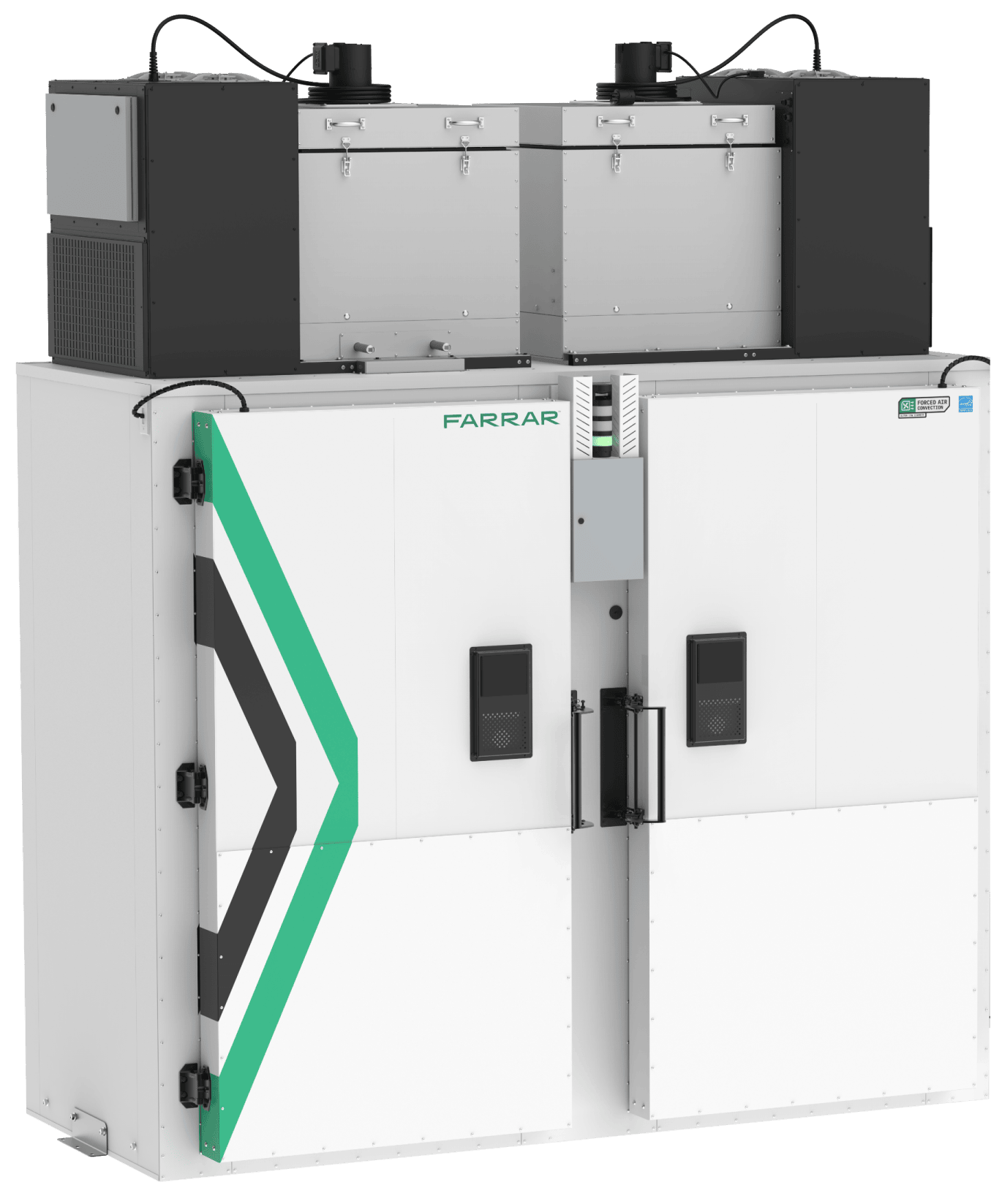
Product ULT Freezer
FARRAR’s Ultra-Low Temperature Freezer is SNAP/EU F-gas-compliant, offering low energy consumption with a low global warming potential (GWP) natural hydrocarbon refrigerant (R290/R170). With a temperature range of -50°C to -86°C with
-
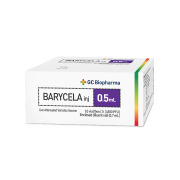
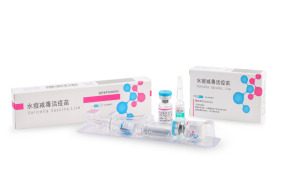
Product Barycela
"World’s 1st Antibiotic-free varicella vaccine" • WHO PQ vaccine • Improved stability with automatic and aseptic process • Over the past 28 years, + 28 M doses provided • Exported more than 30 countires
• Generic name: Varicella Vaccine • Indication: For prophylaxis against varicella • Pr...
-
Product Technology Transfer
Maximize the production capabilities through a de-risking process with our comprehensive technology transfer services for engineering batches, process performance qualification (PPQ) batches, and commercial batches of vaccine antigens, including viral vectors, protein subunits, and virus-like par...
-
Product Vaccine Characterization and Bioanalytical Support
Our vaccines development experts provide a suite of services supporting the analysis and quality control of process and batch release samples and stability studies. We have support the development of a range of vaccines including mRNA, protein, glycoprotein, DNA, carbohydrate, lipopolysaccharide, lipi...
-
Product Pharmatec GmbH Vaccine Manufacturing Line
NEW/NEVER USEDIMMEDIATELY AVAILABLE FROM A CANCELLED PROJECT Complete Pilot Size Vaccine Development Manufacturing Lines Manufacturer: Syntegon Pharmatec GmbH/Germany - 2021
- Pressure Equipment Directive (PED) 2014/68/EU- European GMP (Good Manufacturing Practice) Directives- Vessels pass...
-
Product VET-SAP
VET-SAP® is a veterinary vaccine adjuvant derived from a semi-purified saponin fraction of the Quillaja saponaria tree bark. It is formulated with the purest saponin adjuvant on the market, boasting 90% saponin purity.
Customers can expect consistent saponin purity and profile in every batch, ...
-
Product Varicella Vaccine, Live
It can stimulate the body to produce immunity against varicella-zoster virus, which is used to prevent varicella.

-
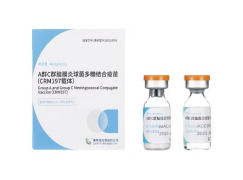
Product Meningococcal Polysaccharide Vaccine (Group A/C/Y/W135)
[Dosage Form]: Freeze-dried powder for Injection [Indication]: The vaccine is indicated for active immunization against invasive meningococcal disease caused by Neisseria meningitis group A, group C, group Y and group W135.
[Specification]: Each 0.5 ml dose of reconstituted vaccine contains 5...
-
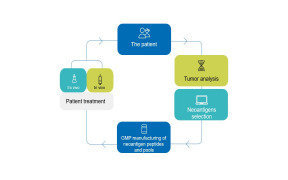
Product NEOANTIGEN PEPTIDES
Our Peptide-based Personalised Medicine Laboratory (PPM Lab) has been designed and equipped with state-of-the-art technology to meet the unique requirements of neoantigens production. A dedicated and experienced team of chemists and doctors ensures full control of the processes.
The implementati...
-
Product 23-valent Pneumococcal PolysaccharideVaccine
Covers a wider range of pneumococcal serotypesMore economical for low-and middle-income countries
Patented process
Great immunogenicity and safety
Prefilled Syringe

-
Product Recombinant COVID-19 Vaccine (Adenovirus Type 5 Vector) Convidecia®
The third-generation technology
Produce humoral and cellular immunity
Stably stored and transported at 2–8°C
WHO Granted Emergency Use Listing (EUL) For Convidecia®
Approved in over 10 countries
-
Product 23-valent Pneumococcal Polysaccharide Vaccine
To prevent invasive diseases caused by 23 serotypes of Streptococcus pneumoniae for use in individuals aged ≥ 2 years who are at increased risk of pneumococcal diseases
-
Product INACTIVATE - I22
INACTIVATE / I22 is a device for the inactivation of pathogens & cells of any kind. By low energy electron irradiation (LEEI) the genetic material of the pathogens & cells is reliably destroyed. The I22 device is suitable for research purposes and small industrial batches. Example use-cases are: Vaccine ...
-
Product CordenPharma MPEG-Conjugated Lipids
CordenPharma also pioneered the chemistry and large-scale manufacture of MPEG-conjugated phospholipids which can be accessed through a synthesis cascade, rendering them superior quality phospholipid examples for sensitive liposome formulation processes.
• MPEG-2000-DMPE - CAS No 384835-59-0 ...
-
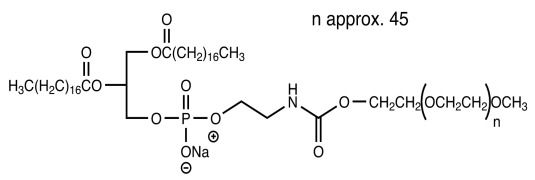
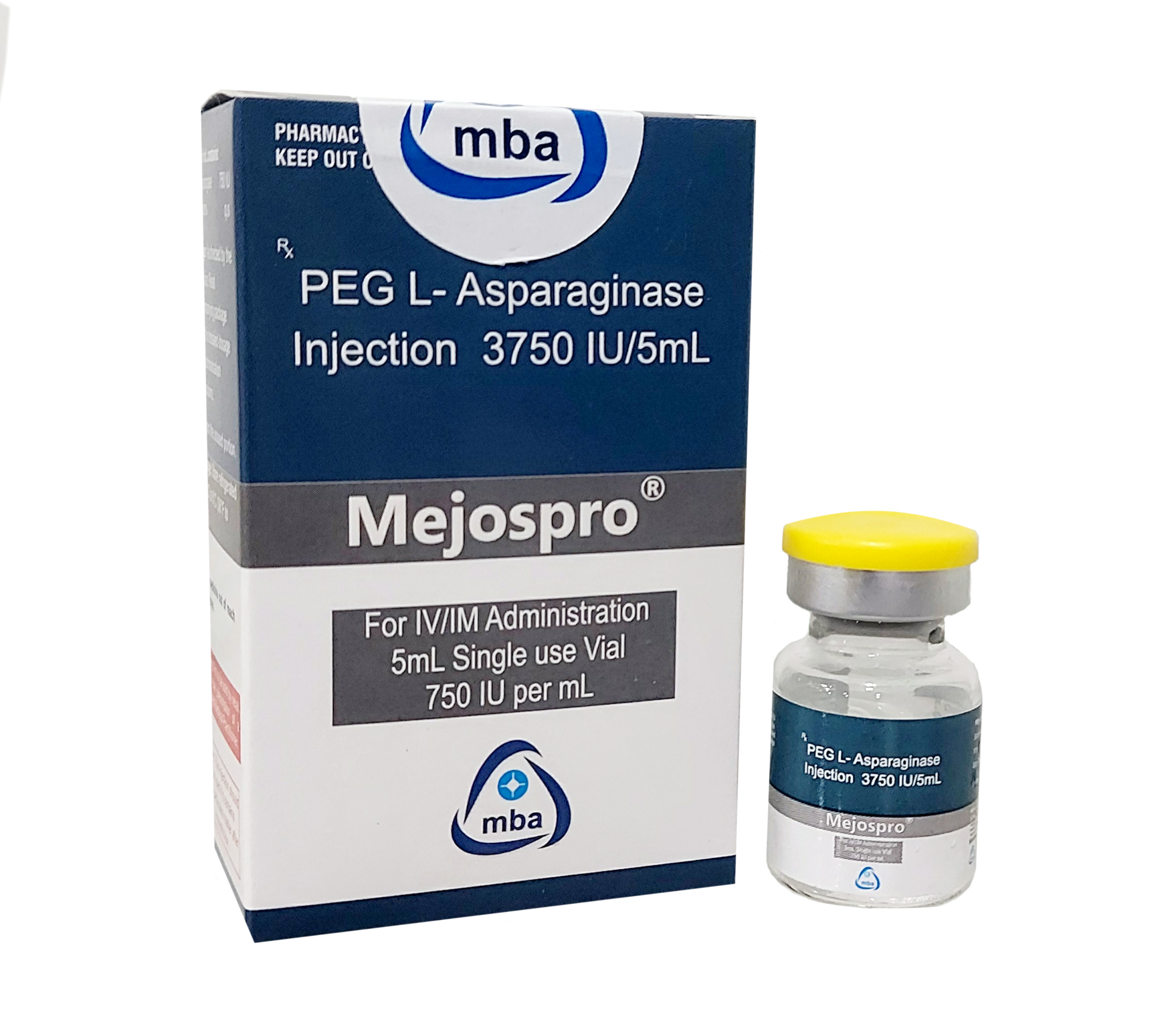
Product MEJOSPRO -Peg L Asparaginase Injection 3750IU/5ML
PEG L-Asparaginase 3750 IU.- Mejospro is used in the treatment of acute lymphoblastic leukemia.
Category: Antineoplastic
This drug is used for :
Peg L Asparaginase is used to treat acute lymphocytic leukemia (ALL).Non-Hodgkin’s lymphoma.Used in some patients...
-
Product DYNO®-MILL UBM (Universal Bead Mill)
Grinding container volume of 0.5 to 100 litres.The name DYNO®-MILL UBM speaks for itself: Universal Bead Mill. It was developed for universal use. The new generation of WAB agitator bead mills covers the entire range from dispersion to ultra-fine grinding. It is suitable for grinding bead diameters fr...
-
Product Adjuvant Systems for Vaccines
We enable next-generation vaccine development through a diverse portfolio of world-leading vaccine adjuvants, immunomodulators and adjuvant formulations.
We passionately develop new adjuvant technologies and partner to develop the vaccines of tomorrow.
Our extensive expertise in immunology and fo...
-
Product Lipid nanoparticles
Lipid nanoparticles (LNPs) enable the delivery of a variety of molecules, including nucleic acids such as mRNA, to cells and are therefore an essential tool in gene therapy. To unleash the potential of vaccines, protein and gene therapies, drug developers need the support of a trusted and experienced pro...
-
Product Process Plant Master Plant
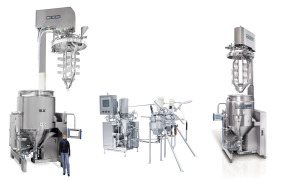
The IKA Master Plant homogenizing and emulsifying plant is a universal mixing plant developed for the production of emulsions and suspensions in the pharmaceuticals industry in particular, but also in the food, beverages, cosmetics and chemical industries. The plant is GMP-compliant and guarantees ...
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

















.png)
.png)


.png)



.png)
.png)
































.png)







.png)

.png)



.jpg)


