7
May
2024

Zhejiang Langhua Pharmaceutical Co., ltd
Exhibitor at CPHI North America 2024 stand 1530
About Us
Categories

-
CN
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
Event information
CPHI North America 2024
-
07 May 2024 - 09 May 2024
-
Pennsylvania Convention Center, Philadelphia
-
Visit us at stand 1530
Products Featured at CPHI North America 2024
-
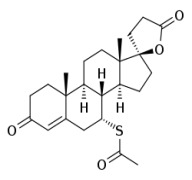
Product Spironolactone
cardiovascular system drugs CEP and USDMF registered -
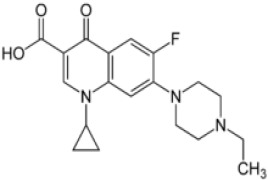
Product Enrofloxacin
Third generation Quinolones antibacterial products, veterinary use CEP and USDMF registered -
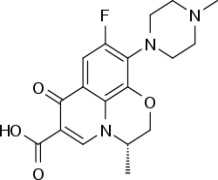
Product Levofloxacin Hemihydrate
Quinolones antibacterial product, anti-infective CEP , USDMF registered -
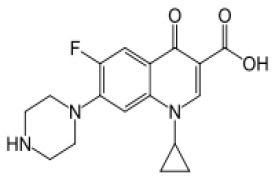
Product Ciprofloxacin Hydrochloride
Wide-range antibacterial -
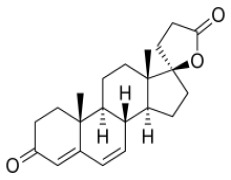
Product Canrenone
Intermediate grade: Assay: NLT 90%; NLT 97%.
API grade
Zhejiang Langhua Pharmaceutical Co., ltd Resources (1)
-
Video Applications of Green Chemistry in API Production
This webinar originally aired as part of CPHI Discover - 17-28 May 2021 Green Chemistry Overview Introduction to Green Chemistry- Twelve Principles and Examples Green Chemistry via Continuous Flow Advantages and Disadvantages Continuous flow reactors Continuous flow chemistry at Langhua
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance