- Home
- Intermediates, Fine and Specialty Chemicals (Products)
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products551,744
-
Companies7,781
-
Articles11,636
-
Events8
-
Webinars342
Intermediates, Fine and Specialty Chemicals (Products)
Intermediates, Fine and Specialty Chemicals (Products) Companies (500+)
Intermediates, Fine and Specialty Chemicals (Products) News
-
News Evotec enters drug discovery collaboration with Janssen Pharmaceutica NV
Evotec and Janssen Pharmaceutica team up to discover novel therapeutic candidates14 Jun 2022 -
News Seqens commits to US market with multi-million dollar investment in R&D
A redeveloped R&D facility will enhance Seqens' capabilities in developing and producing APIs and active delivery systems development23 Mar 2022 -
News Steps for Building Supply Chain Integrity and Resilience in Europe
Speakers from the European Fine Chemicals Group, the voice of fine chemical manufacturers in Europe, led a discussion on the ‘globalised, fragmented and complex’ API and raw materials supply chain, in this month’s edition of the CPHI ...2 Feb 2022 -
News Flamma USA opens doors to HPAPI suite
The CDMO says the suite will help to "bring back manufacturing to the US"5 Nov 2021 -
News API supply chain needs to address future challenges arising from China situation: CPHI Worldwide panel
COVID-19 pandemic has exposed weaknesses and sector faces new challenges driven by rising costs of production in China26 Oct 2021 -
News Next stop, Milan - Welcome to Italy's life sciences hub
Milan is home to an internationally competitive pharmaceutical, biotechnology and functional foods hub, with strong growth prospects and a commitment to innovation across its entire supply chain. Italy is also home to one of the most advanced and ...21 Oct 2021 -
News AGC Pharma Chemicals Europe invites you to a live guided virtual tour
Step inside our state-of-the-art plant to learn about our small molecules CDMO services and meet our team with a virtual tour that focuses on your areas of interest.6 May 2021 -
News CPHI Online Report - Pharma Market Trends 2021
Twelve pharma market trends we anticipate across 2021 for the drug development, manufacturing and outsourcing sectors.25 Nov 2020 -
News Empathy is the key to successful leadership: Women in Leadership Forum
Female life sciences leaders share their experiences and discuss the skills and attributes needed these days to become a successful leader and empower teams22 Oct 2020 -
News CPHI Festival of Pharma Blog
With the Festival of Pharma now well and truly upon us, keep abreast of all that is happening and when over the ten days of this vast virtual event.15 Oct 2020 -
News CPHI Festival of Pharma Podcast - Day One
The CPHI Festival of Pharma has finally opened up! Tune into this podcast in which Informa Markets Head of Pharma Content, Tara Dougal, and Pharma Editor, Gareth Carpenter outline the rich content that is available to registrants on the first day of th...4 Oct 2020 -
News *Ultra Pure Sodium Hyaluronate* by Contipro
Please find company brochure attached that includes the ultra pure specification22 Sep 2020 -
News CPHI South East Asia rescheduled to May 2021
Details of new CPHI South East Asia Online Business Matchmaking Platform to be announced shortly.12 Aug 2020 -
News CPHI Japan rescheduled for Spring 2021
The event organizers are working on preparing bespoke digital product offerings to help the market continue to meet, do business and exchange ideas later this year.27 Jul 2020 -
News CPHI Worldwide to transform into Festival of Pharma digital experience
The 10-day event will feature an interactive digital marketplace for sourcing products and services, enhanced matchmaking for connecting with new and existing partners, and world-renowned speakers.30 Jun 2020 -
News CPHI & P-MEC China moves to December to enable international attendance
Date alteration taken at the request of exhibitors to ensure the international character of China’s largest pharma exhibition.21 Apr 2020 -
News CPHI announces new pharma events calendar for 2020
Organizers secure three new dates for events in Japan, South East Asia and North America in response to COVID-19.17 Mar 2020 -
News Indian pharma supply chain disruption could surpass 2017 if COVID-19 not contained within three months: Ind-Ra
Indian pharmaceutical supply chain disruption could be far greater than the China environmental regulation-induced shortfall of 2017 If the COVID-19 outbreak is not contained within the next three months, credit ratings agency India Ratings and Researc...13 Mar 2020 -
News CPHI Japan 2020 Postponed
Rescheduling will ensure all stakeholders can take part in a successful exhibition and conference.27 Feb 2020 -
News CPHI South East Asia rescheduled for July 2020
Event move ensures extra precautions and safety measures taken18 Feb 2020
Intermediates, Fine and Specialty Chemicals (Products) Products (2500+)
-
Product Sodium Acetate
Alchacet by Alchimica offers high-quality acetates tailored for diverse industrial applications.By prioritizing excellence, quality and reliability, Alchacet ensures that every batch meets thehighest standards of performance. Trust in Alchacet to deliver cutting-edge solutions thatenhance your industrial p...
-
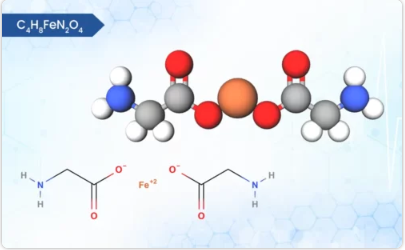
Product FERROUS BISGLYCINATE
Ferrous bisglycinate, a well-tolerated iron supplement, boasts unique physical and chemical properties. It is light to dark greenish powder with occasional friable lumps and virtually no odor. Interestingly, this powder is non-hygroscopic, meaning it won’t readily absorb moisture from the air, enhancing it...
-
Product NH3 MeOH /NH3 EtOH / NH3 IPA / NH3 THF / MMA MeOH / MMA EtOH / MMA H2O / DMA THF
Ammonia, Monomethylamine, Dimethylamine (gas) dissolved in organic solvents at tailor-made concentrations ready to be used in your API and Fine Chemicals synthesis process.
These mixtures will bring less risks (i.e no hazardous gas handling), more productivity (i.e free up space in the reactors)...
-
Product Triphenylphosphine (TPP) [CAS# 603-35-0]
Hokko chemical industry offers a wide range of phosphines products which includes triphenylphosphine. It belongs to organophosphines category. Contact us for more information.
-
Product Ginkgo Biloba Extract
Changsha Huir Biological-Tech Co., Ltd. is a Pre-listed professional herbal extracts and APIs manufacturer with Five Plants in China, Three Warehouses in USA(CA,NY,FL), One warehouse in Europe Changsha Huir Biological-Tech Co., Ltd. Hunan Huirui Pharmaceuticals Co; Ltd Address: Fl 20, Jin...
-
Product DEPOLLUTION WITH NANOSCAVENGERS
We are looking for a strategic environmental partner in order to develop pilot programs on heavy metals, PFAS... specific at your company with the use of magnetic nanoparticles (NP's). Our technological platform allows us to create Nano-Scavangers capable of searching for pollutants in liquids (wastewater,...
-
Product Ticagrelor
CTX Manufacturer Form - II
O DMF available.
CEP Approved.
CTX Lifesciences respects patent laws and conventions of pharmaceuticals as applicable in different countries.
API/Substances covered by patent are not offered to the countrie...
-
Product Gobi Gold Daily Balancer(R)
Incorporating PRT(R) Beads and Fusion Technology(R) in Gobi Gold Daily Balancer(R)enabled us to combine the essential fat-soluble and water-soluble nutrientsin ONE capsule simplifying dosing schedule without exceeding therecommended daily dose. We succeeded to combine materials with different properties an...
-
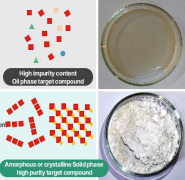
Product TBFA
High purity target compounds in solid phase (amorphous/crystalline form) rather than oil phase.
Detailed technologies: 1) High-purity synthesis technology
2) Separation and purification technology
3...
-
Product Custom and Flow Chemistry
Our continuous flow technology platform enables access to better synthetic strategies which are shorter, offer assured safety, reduce environmental impact and control of by-products. In addition, improved yields and improved reaction selectivity is achievable. With the reaction under tight control, the...
-
Product Bromine and Derivatives
Our Bromine and Derivative products are used in chemical synthesis, oil and gas well drilling and completion fluids, mercury control, paper manufacturing, water purification, beef and poultry processing and various other industrial applications. Other performance chemicals that we produce include tertiary ...
-
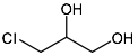
Product 3-Chloro-1,2-propanediol (R)-(-)-Epichlorohydrin (S)-(+)-Epichlorohydrin (R)-(+)-Propylene carbonate
(R)-(+)-Propylene carbonate 16606-55-6 (R)-(-)-Epichlorohydrin 51594-55-9; (S)-(+)-Epichlorohydrin 67843-74-7; 3-Chloro-1,2-propanediol 96-24-2; (R)-(-)-3-Chloro-1,2-propanediol 57090-45-6; (R)-(-)-Glycidyl butyrate 60456-26-0; (S)-(-)-4-Chloromethyl-2,2-dimethyl-1,3-dioxolane 60456-22-6; (S...
-
Product (R)-4-methyl-1,3,2-dioxathiolane 2,2-dioxide 1006381-03-8
- Appearance: Colorless to light yellow solid- Molecular formula: C3H6O4S- Molecular weight: 138.14- Melting point: 81-83°C- Boiling point: 221.8±7.0 °C (Predicted)- Solubility: Soluble in water and organic solvents such as alcohol and ether
-
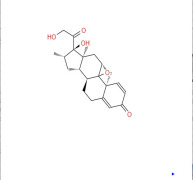
Product 16β-Methyl Epoxide (DB11)
9β,11β-Epoxy-17,21-dihydroxy-16β-methylpregna-1,4-diene-3,20-dione. This compound is a key intermediate in the dexamethasone and betamethasone series of drugs.
-
Product mPEGs
• Methoxy-PEG MW 2K/5K/10K/12K/20K/30K/40K
• 4-arm-PEG MW 10K/20K/40K
• Methoxy-PEG-amine : MW 5K/10K/12K/20K/30K
• Methoxy-PEG-maleimide : MW 5K/20K
• Methoxy-PEG-NHS : MW 2K/5K/10K/12K/2...
-
Product Valsartan
Status: Commercial; Specification: EP,USP;Submission of Regulations:EDMF, CEP, USDMF, CDMF.
-
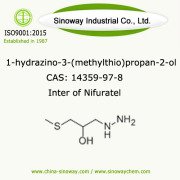
Product Fomepizole [7554-65-6]
Clear colourless to yellow liquid.
It is also known as 4-MethylPyrazole
-
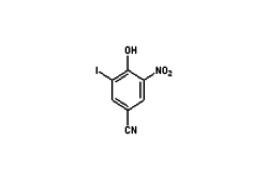
Product 5-Bromoindole-2-carboxylic acid
We are a manufacturer from China, producing all kinds of pharmaceutical intermediates and apis. 5-Bromoindole-2-carboxylic acid is one of our popular products in the world.
Our company also produces and supplies its similar products, such as:
5-Bromo-2-fluoro-m-xylene CAS 99725-44-7&...
-
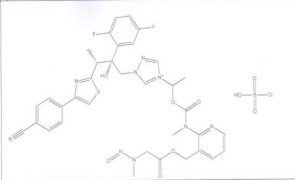
Product N Nitroso Isavuconazonium
N Nitroso Isavuconazonium/ Sulfuric acid
1-((2R,3R)-3-(4-(4-cyanophenyl)thiazol-2-yl)-2-(2,5-difluorophenyl)-2- hydroxybutyl)-4-(1-((methyl(3-(((N-methyl-N-nitrosoglycyl)oxy)methyl)pyridin-2- yl)carbamoyl)oxy)ethyl)-1H-1,2,4-triazol-4-ium salt
-
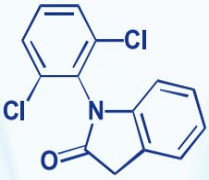
Product Indolinone
CAS NO.:15362-40-0Chemical Name: 1-(2,6-dichlorophenyl)-2-indolinone
Molecular Formula: C14H9Cl2NO
Molecular Weight: 278.13
Usage:Intermediate of Diclofenac Sodium
-
Product Pharma Intermediates
As per customer requirements.
Visit website for detailed product list : https://aparnapharma.com/intermediate/
-
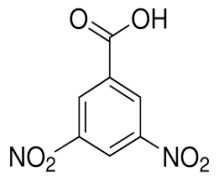
Product 3,5-Dinitro Benzoic Acid
Color : Light Yellow
Form : Crystals
Melting Point : Not less than 206°C
Color of Solution : Less than GY5
Absorbance [410 nm] : Less than 0.95
Loss on Drying (%) : Not more than 0.2
Assay : Not less than 99%

-
Product Uni-Carbomer 20 / Acrylates/C10-30 Alkyl Acrylate Crosspolymer
Discover new possibilities in rheology control with Uni-Carbomer 20 polymer. Improve processing without sacrificing performance, and take your formulations to a higher level. For high clarity, low tack and smooth flow in gels, the choice is Uni-Carbomer 20 polymer. For efficient thickening in the presence ...
-
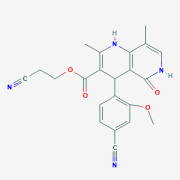
Product 2-Cyanoethyl 4-(4-cyano-2-methoxyphenyl)-2,8-dimethyl-5-oxo-1,4,5,6-tetrahydro-1,6-naphthyridine-3-carboxylate
Finerenone intermediate
Product name: 2-Cyanoethyl 4-(4-cyano-2-methoxyphenyl)-2,8-dimethyl-5-oxo-1,4,5,6-tetrahydro-1,6-naphthyridine-3-carboxylate
Molecular formula: C22H20N4O4
Molecular weight: 404.4
CAS No.: 1050477-43-4
Quality standard...
-
Product Glucono-Delta-Lactone
Package: pe bag lined cardboard drum (size: 37x43), 25kgs net each. Transportation & storage: it is a non-dangerous product and can be transported as a common chemical product. Avoid insolation and rain.keep in dry, clean and ventilating places. The shelf time is 2 years. Contact us for more information.
-
Product Chenodeoxycholic acid
Cholic acid;
Deoxycholic acid;
Dehydrocholic acid;
Dehydrocholate sodium;
Glycocholic acid;Chenodeoxycholic acid;
7-ketolithocholic acid;
Ursodeoxycholic acid;
Tauroursodeoxycholic acid;
Tauroursodeoxycholate sodiu...
-
Product Hydroxypropyl Beta Cyclodextrin
2-Hydroxypropyl beta cyclodextrin: white powder,sweet; increase the solubility of the medicine and biological availability.
CAS No. 94035-02-6(128446-35-5)
-
Product N-Methylaniline (NMA)
N-Methylaniline (NMA) stands as a pivotal compound in the chemical industry, and is known for its broad applications from enhancing gasoline's octane levels to being a key intermediate in manufacturing dyes, agrochemicals and other organic products. It also acts as an effective solvent in organic synthesis...
-
Product Imidazole
CAS No.:[288-32-4]
Molecular formula:C3H4N2
Formula weight:68.08
EINECS No.:206-019-2
Appearance: White crystal
Packing: Inner double PE bag; outer cardboard drum, steel drum or plastic woven bag. Class III package.
Storage conditions: In a cool, ventilated, and dry room, ...
-
Product 4-Hydroxyphenylacetic Acid
Shandong Yangcheng Biotech Co. Ltd offers wide range of pharmaceutical products which includes 4-Hydroxyphenylacetic Acid.
it is a monocarboxylic acid that is acetic acid in which one of the methyl hydrogens is substituted by a 4-hydroxyphenyl group.
Usage: an important pharmaceutical inter...
-
Product Zinc Chloride Anhydrous
(1) catalyst in organic synthesis of pharmaceutical intermediates.
(2) pre-treatment agent in electroplating and galvanizing.
(3) corrosion inhibitor in water treatment.
(4) agent of printing/dyeing in textile.
(5) battery electrolyte.
(6) essential micro-nutrient in fertilizers.

-
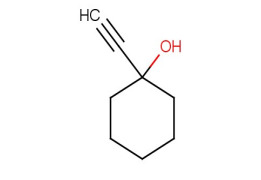
Product 1-Ethynyl-1-cyclohexanol
CAS :78-27-3
CAT# :1896
NAME :1-Ethynyl-1-cyclohexanol
MDL :MFCD00003858
EINECS :201-100-9
MOLWEIGHT :124.18
MOLFORMULA :C8H12O

-
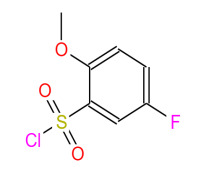
Product 5-Fluoro-2-methoxybenzenesulfonyl chloride
Name: 5-Fluoro-2-methoxybenzenesulfonyl chlorideCas No: 67475-56-3
MF: C7H6ClFO3S
-
Product Fmoc-Amino Acids
Water(K.F)≤1.0 %Any other impurity≤0.1 %Purity (HPLC)≥99.0%(Area%)
Assay(AT)≥98.5%(AT)
-
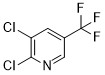
Product 2,3-Dichloro-5-(trifluoromethyl)pyridine
Dalian join king fine chemical co. Ltd offers a wide range of products which includes 2,3-Dichloro-5-(trifluoromethyl)pyridine. Contact us for more information.
-
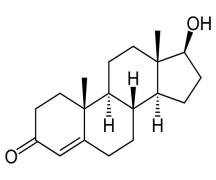
Product Testosterone
Testosterone is the male sex hormone that is made in the testicles. It is used as an alternative treatment of anteschia, also used in treating male climacteric syndrome and erectile dysfunction.Testosterone has CP, EP and USP specification, DMF and CEP is in-process.
-
Product Cetyl-PG hydroxyethyl palmitamide(CAS:110483-07-3)
Cosmetic raw materials
Cetyl-PG hydroxyethyl palmitamide
CAS:110483-07-3
-
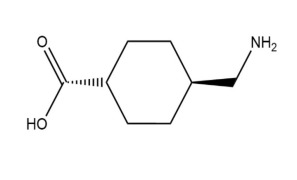
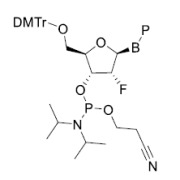
Product 1-[(tert-butoxy)carbonyl]azetidine-3-carboxylic acid
1-[(tert-butoxy)carbonyl]azetidine-3-carboxylic acid
-
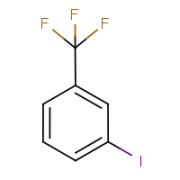
Product 3-Iodobenzotrifluoride
Infinium Pharmachem Manufacturing various Organic and Inorganic Iodine derivatives.
Cas No :- 401-81-0
Molecular Formula :- C7H4F3I
Synonyms :-1-IODO-3-(TRIFLUOROMETHYL)BENZENE 3-IODO-1-TRIFLUOROMETHYLBENZENE 3-IODO-ALPHA,ALPHA,ALPHA-TRIFLUOROTOLUENE 3-IODOBENZOTRIFLU...
-
Product Semi-Solid Solutions
We produce ointments, creams, emulsions, pastes, and gels (including basic components) through our systems. This encompasses both semi-finished and final products, including pharmaceuticals. The manufacturing process, whether automated or semi-automated, depends on the order volume. Hänseler Swiss Phar...
-
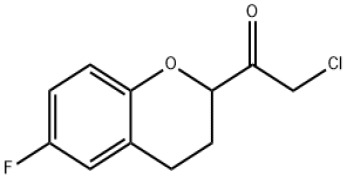
Product 2-chloro-1-(6-fluoro-3,4-dihydro-2H-chromen-2-yl)-ethanone
Aba Chemicals Corporation has all the technology and capability to manufacture at commercial scale the product 2-chloro-1-(6-fluoro-3,4-dihydro-2H-chromen-2-yl)-ethanone (also known as Chloroketone used fas KSM or the manufacturing of API named Nebivolol), also we have the knowledge for the downstream epox...
-
Product (2S)-2-(8-amino-1-bromoimidazo[1,5-a]pyrazin-3-yl)-1-Pyrrolidinecarboxylic acid phenylmethyl ester
CAS NO.: 1420478-88-1Purity: 99%
Documents and Audit can be supported
-
Product Analytical Services
Actylis performs routine and non-routine analytical testing for various industries, in support of manufacturing processes, QA/QC functions, R&D projects, and environmental applications. Leveraging state-of-the-art instrumentation, extensive knowledge base, as well as internal, cross-functional competen...
-
Product Trusted Ingredients for Pharmaceuticals
With Spectrum Chemical, meet the requirements specified in the USP and NF monographs, which are the official standards for all prescription and over-the-counter medicines, dietary supplements, excipients and other healthcare products.
- Ideal for parenteral, oral, topical and ophthal...
-
Product SIMULATED MOVING BED CHROMATOGRAPHY (SMB)
KD Pharma is a contract manufacturer that develops products in the pharmaceutical space by employing state-of-the-art technology. Since its inception in 1988, KD Pharma has focused on utilizing cutting edge technologies to develop and manufacture exceptional quality APIs. These twin pillars of technology a...
-
Product 6-(4-Aminophenyl)-4,5-dihydro-5-methyl-3(2H)-pyridazinone
Application: Levosimendan intermediate, the purity is 99.9%, maximum single impurity is 0.07%, off-white solid.

-
Product (R)-(-)-3-Quinuclidinol
Arran offers a wide range of chiral products which includes (R)-(-)-3-Quinuclidinol. Contact us for more information.
-
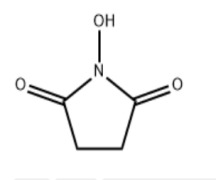
Product N-Hydroxy succinimide
Appearance: white crystalline powder Assay: ≥99.0%(by HPLC)
Usage: it can be used in the synthesis of amino acid protective reagents, and can also be used as a pharmaceutical intermediate.
-
Product WeylChem InnoTec > Innovative Fine chemical, Pharmaceutical and Electronic Industry Partner
WeylChem InnoTec are your innovative partner to the fine chemical, pharmaceutical and electronic industry. We provide world-class analytical, modern developmental and tailor-made manufacturing services. With our competencies in Analytics, Contract Development & Custom Synthesis, WeylChem InnoTec is you...
-
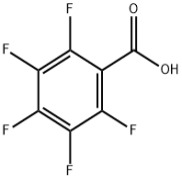
Product 2,3,4,5,6-Pentafluorobenzoic acid
We offer a wide range of product which includes 2,3,4,5,6-Pentafluorobenzoic acid. Contact us for more information.

-
Product 2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyriMidine-6-carboxylic acid
Raw materials Ribociclib intermediate
-
Product Chemicals / Intermediates for Pharma & Biopharma
Since its foundation, Midas Pharma has been offering services in sourcing and supplying of substances complying with the corresponding industry requirements and uses. Meanwhile our portfolio involves a broad range of chemicals for industrial applications, starting materials and intermediates for small mole...
-
Product Lithium aluminum deuteride
The pure substance of this product is white grey powder. Melting point:125℃, it is stable under normal pressure and temperature. It can be hydrolyzed in moist air. It must be stored under the inert gas at a cool, dry ventilated place.
-
Product Methylnitroguanidine 1-Methyl-3-Nitroguanidine N-Methyl-N-Nitroguanidine
It is an intermediate of organic synthetic insecticide and pharmaceutical, is mostly used to produce 3-Methyl-4-Nitroiminoperhydro-1,3,5-Oxadiazine.
-
Product Ethisterone
Hubei Sanjing Biotechnology Co Ltd offers a wide range of products which includes ethisterone. Contact us for more information.
-
Product Vaccine Adjuvant: Alhydrogel™
• Alhydrogel is a range of aluminium hydroxide gel products which have been specifically developed for use as an adjuvant in human and veterinary vaccines • The gel is a suspension of boehmite-like (aluminium oxyhydroxide) hydrated nano/micron size crystals in loose aggregates • The products have v...
-
Product CAS 1143516-05-5
We have more than 100kg available goods in stock, and this product is in pilot scale. The purity can reach more than 98.0%.

-
Product Sodium saccharin 6% moisture
JMC offers a wide range of products which includes sodium saccharin 6% moisture. Appearance: it is white crystals or a white, crystalline powder or colorless crystals. Packaging: 25kg carton box, 50kg fiber drum, and 1000kg super sack with inner polyethylene bag. Contact us for more information.
-
Product 2-Hydroxy methyl 3-methyl-4-[2,2,2 trifluoroethoxy] pyridine hydrochloride
INTEGRIN LIFE SCIENCES PVT. LTD. offers wide range of Intermediates which includes 2-Hydroxy methyl 3-methyl-4-[2,2,2 trifluoroethoxy] pyridine hydrochloride (Lanso Hydroxy). Contact us for more information.
-
Product R-(-)-3-Quinuclidinol
CAS: 25333-42-0The company’s product range, which is constantly being developed and expanded, includes 60 finished dosage forms, 25 active pharmaceutical ingredients and more than 20 intermediates, including medicines for the nervous system, cardiovascular health, as well as antiviral, antibacterial and...
-
Product Phenyl Acetone
We are a manufacturer of the said product in India. For all further details please visit our website www.premierindia.co.in or email us:- [email protected]; [email protected]
-
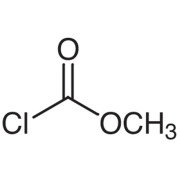
Product Methyl chloroformate
Specification
Appearance : Transparent liquid
Transparent yellow liquid
Purity : 98.0% min.
Acid : 0.2% max.
Packing
200Kgs net PE lined chemical drum (UN marked).

-
Product Sodium Hyaluronate (Hyaluronic Acid API): MW 7-250 kDa
Sodium Hyaluronat (Hyaluronan, HA): TYPE II (MW 7-250 kDa)
Very Low Molecular Weight - Pharmaceutical Grade (Injection suitable)Certificates: DMF, GMP, USP, EP
Sodium hyaluronate is a polysaccharide found in many locations in the human body, such as t...
-
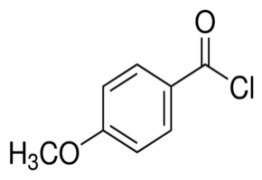
Product 4-Methoxybenzoyl chloride
4-Methoxybenzoyl chloride is one of the reactive acylating agents that can react with carboxylic acids, alcohols and amines to yield respective carboxylic anhydrides, esters and amides.
Shree Ganesh Remedies Limited is one of the leading manufacturer and supplier of 4-Methoxybenzoyl chloride [C...
-
Product Generic APIs, biocides and intermediates
An extensive portfolio of generic APIs and intermediates renowned for their quality, safety and supply securityEvonik is one of the world’s leading suppliers of high-quality generic APIs, biocides and intermediates for the pharmaceutical industry. As a global provider for generic APIs, we offer a broad por...
-
Product ETHYL ACETATE
Ethyl acetate used for pharmaceutical industry as an intermediate and solvent for crystallization. Has a high solvent power, compatible with all solvents, thinners, plasticizers, common resins. Dollmar has full availabilty of ethyl acetate in drums, ibc and bulk. https://www.dollmar.com/cms/?dollmarproduct...
-
Product DISULFIRAM
CODE: QF0104SYNONYM: Bis(diethylthiocarbamoyl)disulfide
CAS: 97-77-8
MOLECULAR FORMULA: C10H20N2S4
-
Product 1,2-Ethanedithiol 540-63-6
Appearance: Colorless transparent liquidAssay: 98.5%min
Refractive index: 1.550 to 1.560
BP: 144degC to 146degC
Density: 1.120 to 1.124g/ml
-
Product Outsourcing of advanced intermediates, active ingredients and formulations
With an extensive range of products and world-wide experience, Summit Pharmaceuticals Europe is able to provide integrated services for the pharmaceuticals industry, from raw materials and APIs up to finished dosage forms.

-
Product 2-CHLOROMETHYL-3,4-DIMETHOXY PYRIDINE HCL
PALECREAM COLOR CRYSTAL POWDER
300t/ year
Contact us for more information

-
Product Expert witness and intellectual property support
Intertek offers wide range of pharmaceutical services which includes expert witness and intellectual property support for pharmaceuticals. It belongs to pharmaceutical and healthcare consulting services category. It includes expert witness and intellectual property support services, intellectual property s...
-
Product POVIDONE, MICROCRYSTALLINE CELLULOSE, HYPROMELLOSE, CROSPOVIDONE, COPOVIDONE, CCS, COLLOIDAL MCC, LACTOSE, STARCH, SUCRALOSE, SACCHARIN, CYCLODEXTRINS, POLOXAMER, POLYSORBATE, PEG, API, INTERMEDIATES, FILTER BAGS, SILICA, SILICONES,
DILUENTS, BINDERS, DISINTEGRANTS, FILM FORMERS, PLASTICIZER, POLYMERS, SWEETENERS, SURFACTANTS, PEPTIDES, OMEGA 3 FATTY ACID, VITAMINS, DIETARY FIBRES, COCOA POWDER, ANTI AGEING, ANTI WRINKLES, MOISTURIZERS, ENCAPSULATED BEADS, SCRUBING BEADS,
-
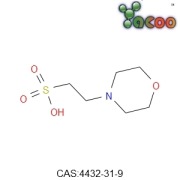
Product MES
Specification:
English Name:2-Morpholinoethanesulfonic acid
Molecular Formula:C6H13NO4S Molecular Wt:195.23
CAS:4432-31-9
Usage:
MES is biological buffer, this product is suitable for the soap and soap, can be used as dispersant of calcium soap. Is also the...
-
Product HEXAMETHYLDISILAZANE
• 26 years of professional product experience, original solvent-free patented technology,the first drafter of group standards
• Premium quality globally, with a content of 99.9%, specific impurity 0.1 PPB Max., providing customized products
• Leading producer globally, 7,000 t/a capaci...
-
Product tert-Butyl 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-5,6-dihydropyridine-1(2H)-carboxylate
Product Name:tert-Butyl 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-5,6-dihydropyridine-1(2H)-carboxylateCAS NO :885693-20-9
Moluculer Weight :309.20
MDL NO :MFCD10697911
Purity/Specification:97%
Smile Code :O=C(N1CCC=C(B2OC(C)(C)C(C)(C)O2)C1)OC(C)(C)C
Boiling Point :348°C at 760 mmHg qq...
-
Product (R)-1-tert -Butoxycarbonyl-3-aminopyrrolidine (R-BAMP)
・Batch scale:150kg/Batch
・Purity:over 98.5%
・Optical purity:over 99.0%ee

-
Product 4,4'-Diiodobiphenyl
Tosoh provides a wide range of iodinated products which includes 4,4-diiodobiphenyl. It belongs to fine chemicals category. Contact us for more information. -
Product DSC; CAS# 74124-79-1 Peptide Coupling Reagent
Coupling Reagents of peptides; Purity(99% HPLC): 99%min;
-
Product NITROXYNIL
CAS No.: 1689-89-0Indication: Anthelmintic Category: Veterinary API Specifications: As per BP.VET. Description: We provide Nitroxynil which is perfectly suited for use in all dosage forms such as oral suspension, Injections and drenches with excellent stability & consistency.
-
Product Intermediates, Phase Transfer Catalysts, Advance Intermediates, APIs, Contract Manufacturing
We are manufacturer of advance intermediates such as meta bromo anisole and Schiffs base of Tramadol Hydrochloride and Sertraline hydrochloride respectively. We also provide TBAB, TBAC, ABAI and other Phase transfer catalysts in tons scale.
-
Product Pharmaceutical Grade heavy magnesium Carbonate
Details
1,Chemical Name: Pharmaceutical Grade Magnesium Carbonat
2,Molecular Formula: xMgCO3·yMg(OH) 2·zH2O
3,CAS: 39409-82-0
4,Character: Friable masses or bulky, white powder, odorless. Relative density is 2.2. Melting point is 350℃...
-
Product "Ozagrel methylester "
Product Name Ozagrel methylester
Synonyms methyl (2E)-3-[4-(1H-imidazol-1-ylmethyl)phenyl]prop-2-enoate,2-propeno; Methyl (2E)-3-[4-(1H-imidazol-1-ylmethyl)phenyl]acrylate; Ozagrel methylester CAS RN 866157-50-8 Specification ≥98.0% Mo...
-
Product Purified Talcum IP/BP/USP
Neelkanth Minechem is Manufacturer of a wide range of products which include Purified talcum I.P, Purified Talcum B.P, Talc U.S.P, Pharmaceutical grade talcum powder "Puremax" and Cosmetic grade talcum powder "Cosmeta".
-
Product Phloroglucinol dihydrate
Shouguang fukang pharmaceutical co. Ltd offers a wide range of products which includes phloroglucinol dehydrateit is white or wheat rime dust. Contact us for more information.
-
Product (-)-adrenaline ar
S.D. Fine Chem Limited offers a wide range of products which includes (-)-ADRENALINE AR5g. Contact us for more information.
-
Product N-Methyl-Pyrrolidone
Jiaozuo zhongwei special products pharmaceutical co. Ltd offers a wide range of product which includes n-methyl-pyrrolidone. Properties: it is colourless transparent liquid,it has strong water absorption. It can almost dissolve in all the solvents.
Uses: it is a high-selective polar solvent a...
-
Product Ferrous Gluconate
We offer a wide range of products which includes Ferrous Gluconate.
Characteristics: light yellow gray or yellow granule or powder with slight gray green.
Uses: iron is one of the major composition, which constitutes, hemoglobin, myoglobin, cellular chromatin and some tissular enzym...
-
Product Product List of intermediates
Acalabrutinib
Acyclovir
Abemaciclib
Avibactam
Avatrombopag
Apalutamide
Brivaracetam
Baloxavir
Baricitinib
Crisaborole
Copanlisib
Eluxadoline
Dolutegravir
Dapagliflozin
Elagolix
Efinaconazole
Eltrom...
-
Product (1s, 2s, 3s, 5s)-3-(Benzyloxy)-5-(6-(Benzyloxy)-2
Honour Lab Limited offers a wide range of products which includes (1s, 2s, 3s, 5s)-3-(benzyloxy)-5-(6-(benzyloxy)-2-((4-methyl diphyenyl methylamino)-9 hpurin-9-yl)-2-(benzyloxymethyl) cyclopentanol. It belongs to intermediate category. Contact us for more information.
-
Product (2R,3S)-3-phenyl Isoserine Methyl Ester 131968-74-6
Hangzhou longshine bio-tech co ltd offers a wide range of products which includes (2R,3S)-3-phenyl Isoserine Methyl Ester 131968-74-6. It belongs to intermediate category. Contact us for more information.
-
Product 7-amca
Zhejiang east-asia pharmaceutical co. Ltd offers a wide range of products which includes 7-amca. It belongs to intermediate category. Contact us more information.
-
Product Edenor(R) G 99,8 PH
Glycerine Ph. EUR / USP as pharmaceutical excipient certified according to Exipact GMP / GDP. Please contact us for more Information.
-
Product Anhydrous Glucose
Jingjing Pharmaceutical Co., Ltd offers a wide range of products which includes Anhydrous glucose. Function and use: It is used to reduce intraocular pressure and increased due to intracranial pressure caused by various diseases such as cerebral hemorrhage, skull fracture, uremia and so on. Contact us for ...
-
Product Perfluorobutanesulfonyl fluoride
Time chemical provides wide range of products which includes diphenyl-(trifluoromethyle)-sulfonium trifluoromethancesulfonate. Packaging: 25kg/drum. Contact us for more information.
-
Product Avibactam Sodium
Zhejiang Yongning Pharmaceutical Co. Ltd offers a wide range of products which includes Avibactam Sodium. It belongs to APIs category. Storage: cool and dry , protected from light. Contact us for more information.

-
Product Gliclazide tablets
Bal pharma ltd offers a wide range of products which includes Gliclazide tablets. It belongs to Antidiabetic range. Composition: 80mg, MR 30mg, MR 60mg Tab. Contact us for more information.
-
Product Iodides
Sodium Iodide (NaI) - CAS 7681-82-5
Copper Iodide (CuI) - CAS 7681-65-4
Potassium Iodide (KI) - CAS 7681-11-0
-
Product Pharmaceutical Intermediates
Erythromycin Thiocyanate, 7-Aminocephalosporanic Acid (7-ACA), 7- Aminodeacethoxylcephalosporanic Acid (7-ADCA), AE Active Ester (Benzothiazolium Thioester), Thiotriazinone, DM (Ethyl Acetoacetate), 6-Aminopenicilanic Acid (6-APA), Sodium Borohydride, Potassium Borohydride, Liquid boron, Sodium Hydride, So...
-
Product Nucleotides, Nucleotide triphosphates (NTPs) & Deoxynucleotide triphosphates (dNTPs)
We develop a broad range of Nucleotides, Nucleotide triphosphates (NTPs) and Deoxynucleotide triphosphates (dNTPs).
Nucleotide diphosphates:
Uridine 5'-diphosphate disodium salt (UDP);
Guanosine 5'-diphosphate disodium salt (GDP);
Cytidine 5'-diphosphate disodium salt (CDP...
-
Product Sodium Borohydride
Sodium Borohydride is a very strong reducing agent used in organic and biochemical reactions. Nanocrystalline superlattices in gold colloid solution have been prepared by ligand-induction using AuCl3reduced with sodium borohydride. Nucleophilic addition of hydride ion from sodium borohydride is an inexpens...
-
Product Ethyl cyanoglyoxylate-2-oxime
Zhejiang Hisoar pharmaceutical is global manufacturer and supplier of Coupling Reagent for Peptide .Contact us for more information.
-
Product Sulfadoxine
Chongqing Kangle Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes linocaine base. It belongs to intermediate for api categoryproperties: white needle-like crystals. Soluble in alcohol, ether, benzene, chloroform and oils, insoluble in water. Use: for quetiapine fumarate...
-
Product Ezetimibe
Zhejiang Tianyu Pharmaceutical Co. Ltd offers a wide range of product which includes (s,e)-methyl 2-(3-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-hydroxypropyl)benzoate (intermediate of montelukast). It belongs to pharmaceutical intermediates category. Contact us for more information.
-
Product Nintedanib Esylate
Kekule pharma offers a wide range of intermediates which includes Nintedanib Esylate. Contact us for more information.
-
Product 1-bromo hexane
Yancheng Longshen Chemical Co.,Ltd offers a wide range of products which includes 1-bromo hexane. It belongs to Halogenated Hydrocarbons category. Appearance: Clear or buff liquid. Contact us for more information.
-
Product SUZETRIGINE
Suzetrigine(VX-548) is an orally active and specific NaV1.8 inhibitor. Suzetrigine has analgesic activity and can be used to study acute pain and neurotransmission.As compared with placebo, VX-548 at the highest dose (a 100-mg oral loading dose of VX-548, followed by a 50-mg maintenance dose every 12 hours...
-
Product 3,4,5-Trimethoxybenzoic acid methyl ester
Name: 3,4,5-Trimethoxybenzoic acid methyl ester
Alias: Trimethoprim impurity H
Molecular Formula:C11H14O5;
Molecular Weight: 226.2259
Usage: It is used as a medical intermediate which is the main raw material of anti-anxiety drug tr... -
Product N-Methylglucamine
Diatrizoic Meglumine, Flunixin Meglumine, Methylglucamine Antimonate intermediate

-
Product 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride ( EDC-HCl )
Changzhou Foreign Trade Corp offers a wide range of products which includes 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride ( EDC-HCl ). Contact us for more information.
-
Product 2,3,4,6-Tetra-O-Benzyl-D-Galactose
Characteristics: It is white or off-white solid, it is insoluble in water ,but easily soluble in chloroform.
-
Product Advanced Intermediates
Within our Advanced Intermediates portfolio, we provide fine chemicals and advanced intermediates, such as pyridines and piperidines, Ketene derivatives, phase-transfer catalysts, fluorine compounds, and others, as well as customer-specific products, for a wide range of applications and market segment...
-
Product Pemetrexed Disodium Heptahydrate
Suzhou Lixin Pharmaceutical Co Ltd offers a wide range of products which includes 1,2,3-tri-o-acetyl-5-deoxy-d-ribofuranose. It belongs to intermediates category. Contact us for more information.
-
Product 6,7-Bis-(2-methoxyethoxy)-4(3H)-quinazolinone
Neostar offers a wide range of products which includes 6,7-Bis-(2-methoxyethoxy)-4(3H)-quinazolinone. It belongs to apis intermediates category. Contact us for more information.
-
Product Telmisartan
Jiangsu zhongbang pharmaceutical offers a wide range of products which includes 2-chloronicotinic acid. It belongs to Intermediate Category. Usage: NevirapineIntermediates. Contact us for more information.
-
Product Canrenone
Zhejiang shenzhou pharmaceutical offers a wide range of products which includes Canrenone. Contact us for more information.
-
Product 2,4-Xylidine
Hebei dapeng pharm and chem oc. Offers a wide range of products which includes 2,4-Xylidine. It belongs to dimethylaniline category. Appearance: Colorless or light brown liquid. Packing: The 200KG/steel barrel. Contact us for more information.
-
Product (S)-4-phenyl-2-oxazolidinone(SPOZ)
Changzhou united chemical co. Ltd offers awide range of products which includes (S)-4-phenyl-2-oxazolidinone(SPOZ). It belongs to fine chemical & chrial reagents category. Contact us for more information.
-
Product DL-Carnitine HCl
Liaoning Koncepnutra Co., Ltd. offers wide range of pharmaceutical products which includes dl-carnitine hcl. Characteristic: white powder, it is very soluble in water. Applications: mainly suitable for feed products. Packing: 10kg/ 25kg fiber drum. Contact us for more information.
-
Product BROMINE DERIVATIVES, PHASE TRANSFER CATALYSTS, Intermediates, Lithium Compounds
BROMINE DERIVATIVES Liquid Bromine N-Propyl Bromide Sodium Bromide Powder / solution 45% N-Butyl Bromide PHASE TRANSFER CATALYSTS Tetrabutylammonium Bromide (TBAB) Triethylbenzylammoniun Chloride (TEBAC) Tetrabutylammonium Hydrogen Sulphate (TBAHS)LITHIUM COMPOUNDS Lithium Bromide Lith...
-
Product (-)-2-[4-[(4-chlorophenyl)phenylmthyl]-1-piperazinyl]ethanol dihydrochloride salt
Hunan warrant pharmaceutical co., ltd offers a wide range of products which includes (-)-2-[4-[(4-chlorophenyl)phenylmthyl]-1-piperazinyl]ethanol dihydrochloride salt. It belongs to chemicals and main intermediates category. Contact us for more information.
-
Product 1-(3-Methyl-1-Phenyl-5-Pyrazolyl)Piperazine
Pure Chem Private Ltd offers a wide range of products which includes 1-(3-methyl-1-phenyl-5-pyrazolyl)piperazine. it is api intermediates useful for teneligliptin. Contact us for more information.
-
Product (3S,4S)-3-((R)-(tert-Butyldimethyl-silyloxy)ethyl)- 4((R)-carboxyethyl)-2-azetidinone 4-BMA
Zhejiang jiuzhou pharmaceutical co. Ltd offers a wide range of products which includes (3s,4s)-3-((r)-(tert-butyldimethyl-silyloxy)ethyl)- 4((r)-carboxyethyl)-2-azetidinone 4-bma. It belongs to intermediates category. Contact us for more information.
-
Product CIL Products
CIL specializes in the process of labeling biochemical and organic compounds with highly enriched, stable (nonradioactive) isotopes of carbon, hydrogen, nitrogen and oxygen. Our chemists substitute common atoms (e.g., 1H, 12C, 14N, 16O) with rare, highly valued isotopes (e.g., 2H o...
-
Product Fmoc-L-Dab(Boc)-OH
Specific amino acid form used in peptide constitution.
From our Korean Partner Amino logics Co., Ltd.

-
Product Phosphorus Pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula P2S5 or dimer P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in carbon disulfide but reacts with man...
-
Product Potassium Phosphate Dibasic Pharma Pure, USP/EP/BP Powder
Potassium Phosphate Dibasic can be used as a dietary ingredient and as a nutrient. This product is known to be used in pharmaceuticals, in oral care products, as a laboratory reagent, and in fermentation and cell cultures. It is White odorless powder. Packaging: 55lb/25kg cartons; 110lb poly...
-
Product 2-Phenylethylisocyanate
Synthesia a.s. offers a wide range of products which includes 2-phenylethylisocyanate. Features: it is colourless to yellowish translucent viscous liquid with pungent smell, lachrymatory. Contact us for more information.
-
Product Demeclocycline Hydrochloride
Application: Treatment of Lyme Disease, Periodontitis and Bronchitis
Specification: USP, EP
DMF Status: US DMF, EU DMF
Standard Package: 50kg, Fiber Drum

-
Product Iodine
The largest economically exploitable reserves of Caliche Ore in the world are located in northern Chile, where SQM holds a major part of them. SQM also has the world's largest iodine production capacity. Main uses of iodine: biocides, LCD/LED polarizing films, human nutrition, pharmaceuticals. -
Product 2,4-dichlorobutyrophenone
2,4-dichlorobutyrophenone is an intermediate with use as a key material in the manufacture of pharmaceutical APIs like Penconazole and in the manufacture of further intermediates. Please contact us for more information about specifications, regions of sale, current availability and other applications.
-
Product D-cloprostenol
Giellepi Pharmaceuticals Srl offers a chemical intermediates & api products which includes d-cloprostenol . It belongs to Veterinary chemical intermediates category. Contact us for more information.
-
Product Homoveratric Acid
Recordati S.p.A provides a wide range of intermediates which includes homoveratric acid. Contact us for more information.
-
Product Thiopental
SCI Pharmtech, Inc. offers a wide range of active pharmaceutical ingredients which includes thiopental. Contact us for more information.
-
Product methyl(R)-5-bromo-6-(3-hydroxypyrrolidin-1-yl)nicotinate (CAS#1492885-31-0)
methyl(R)-5-bromo-6-(3-hydroxypyrrolidin-1-yl)nicotinate (CAS#1492885-31-0)
-
Product Drug Substance Manufacturing
Ajinomoto Bio-Pharma Services offers comprehensive CDMO capabilities for small, medium and large molecule APIs, including high potency, ADCs and oligonucleotides. Our global network provides the adaptive solutions, responsive service, trusted partnership and peace of mind you’ve come to rely on. That's...
-
Product 3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride
Sitagliptin Phosphate intermediates,the final API is used for DPP-4 inhibitor,treat Type 2 diabetes.CAS NO. :762240-92-6

-
Product 6-amino-penicillanic acid (6-APA)
Centrient Pharmaceuticals provides wide range of intermediates which includes 6-amino-penicillanic acid (6-apa). Characteristics: a crystalline powder manufactured by an enzymatic process starting from penicillin g potassium. Application: the product is used as an intermediate for the manufacture of s...
-
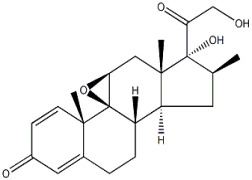
Product 6α-methyl prednisolone
Jiangsu jiaerke pharmaceuticals group co offers a wide range of products which includes 6α-methyl prednisolone. Contact us for more information.
-
Product Lamivudine Intermediate CME
Shijiazhuang Lonzeal Pharmaceuticals Co Ltd offers a wide range of products which includes lamivudine intermediate cme. It belongs to antiviral drugs intermediates category. APIplication: antiviral intermediates. Contact us for more information.
-
Product (S)-2-Aminomethyl-N-Ethyl Pyrrolidine
Levosulpiride Intermediate. Supplying to all pharma companies in India and abroad.
-
Product 2-Amino-2-Phenyl Butyrate Sodium
Benzo Chem Industries Pvt. Ltd offers wide range of Pharmaceutical Intermediates which includes 2-amino-2-phenyl butyrate Sodium . Appearance: white to off white solid . Contact us for more information.
-
Product Potassium Citrate
Canton Laboratories Pvt Ltd. offers various grades. Please contact for details.
What is a pharmaceutical intermediate?
Pharmaceutical intermediates are fine chemicals of intermediate compounds that are produced in the process of manufacturing active pharmaceutical ingredients (APIs). Intermediates are the by-products of reactions in the API production process. Each reaction during the production process may produce several intermediates depending on the analysis, and these intermediates can serve as precursors to other active pharmaceutical ingredients. The conversion of intermediates to active ingredients can be achieved through further refinement processes. Like APIs, intermediates can also be used in bulk drugs for therapeutic purposes.
Difference between an API and an Intermediate
How does an API differ from a pharmaceutical intermediate? These terms may be confused and used interchangeably, especially by newbies in the pharmaceutical industry. Although APIs and intermediates are both pharmacologically active, they differ in function, composition and structure.
What are the major differences between API and Intermediates?
Many factors and analysis differentiate active ingredients and chemical compound from pharmaceutical intermediates. Active ingredients and speciality chemicals are the final products of raw materials, while chemical intermediate make up the by-products produced during the manufacturing process of APIs. Unlike APIs, which are usually safe and administered for therapeutic purposes after due research, intermediates may either be therapeutic, toxic or even APIs. However, APIs produced as by-products that are not officially approved, cannot be administered or used as active ingredients. Also, intermediates do not need to be approved or regulated to be utilized, unlike active ingredients that contain specialty chemical.
What is API in pharmaceuticals?
APIs – Active pharmaceutical ingredients, are the active substances or combination of active substances contained in drugs, that produce the intended pharmacological, biological or therapeutic effect. They serve as the active ingredient of drugs and provide pharmacological activity, or aid diagnosis, treatment, cure, mitigation, or prevention of diseases. Drugs are primarily made up of active pharmaceutical ingredients as the core, and excipient which are either inert or biologically inactive.
Innovations in pharmaceutical intermediates
The pharmaceutical industry is continuously evolving and reshaping itself. Several pharmaceutical companies and market players are adapting innovations in pharmaceutical intermediate sales as the demand for them is rapidly increasing. The growing demand is as a result of several factors such as the surge in chronic diseases, etc., and this has positively impacted the global pharmaceutical intermediate market size. As drug making activities increase around the globe, innovations are being birthed, and the demand for pharmaceutical intermediates manufacturers is on the rise. Due to the implementation of regulated drug-making activities, good manufacturing practices (GMP) in pharmaceutical companies, the global pharmaceutical and packaging market has experienced a surge.
What trends have driven innovations in pharmaceuticals intermediates?
Based on reports, in 2018 forecast period, the global pharmaceutical intermediates market share had an estimated gross margin value of US $ 27, 356.1 million. Its estimated value is anticipated to reach US$38,457.2 million by 2024, with a CAGR of 6.1%, during the 2019-2024 forecast period. Top manufacturers are continually making massive investments into pharmaceutical researches and the development of new pharmaceutical intermediates. Alongside other trends, this gives the market an added advantage.
Some other significant trends driving innovations and growth rate of the global pharmaceutical market size include the rising demand for generic drugs, increasing prevalence of chronic and terminal diseases, patent expiry, and the increasing production of APIs, and intermediate operation among others.
How have pharmaceutical intermediates developed?
Given the massive growth of the market, investments into research, and the continuously advancing biotechnology, pharmaceutical intermediates have experienced some significant developments over the past few years. New intermediates are being discovered, as well as new product development and manufacturing processes such as continuous flow process. These discoveries have made intermediates safer and potent for consumption and have also established new scopes of application.
What are the challenges to pharmaceutical intermediates market growth?
Just as there are key trends motivating innovations and growth of the global pharmaceutical intermediate market, there are also trends that present as challenges. Strict regulatory policies for one, serve as significant setbacks to the growth rate of this market size. Also, strong competition between companies in the industry as well as pricing challenges are adversely affecting the growth of the global pharmaceutical intermediate market. Also, in recent times, the COVID-19 pandemic has also affected pharmaceutical intermediates sales and market share growth.
Top manufacturers of pharma intermediates
The pharmaceutical ingredient market share has a broad pool of competitor pharmaceutical companies at different levels. However, some have strategically worked their way to becoming a top API manufacturer of pharma intermediates in the pharmaceutical industry. These leading manufacturers have played vital roles in the growth of the global intermediate market through stocks. Some of these manufacturers include Aceto Corporation, Vertellus Holdings LLC, Dishman Group, Cycle Pharma, Sanofi Winthrop Industries SA, BASF SE, Teva Pharmaceuticals, AR Life Sciences Private Limited, Midas Pharm etc.
Where can you find the top manufacturers of pharma intermediates?
Although pharmaceutical intermediates are manufactured in several countries and regions across the globe, there are focal points where top manufacturers are located. These regions contribute some of the largest quotas to the growth of the intermediate pharmaceutical market. The major regions include Asia Pacific, North America, Latin America, MEA, and Europe, with the expectance that North America will contribute the largest to the market revenue in the near future.
In addition to this, Asia Pacific and Latin America are also expected to influence the market's growth positively. There are anticipations that top manufacturers in Japan, China, and India will also impact market growth positively.
What do the top manufacturers of pharma intermediates have in common?
Most top players in the pharma intermediate global market have a lot of things in common, keeping them at the top of the supply chain and contributing to the growth rate of the worldwide market. Top manufacturing companies of pharmaceutical intermediates make massive investments into innovations, research, and new pharma intermediate development. They take advantage of recent market trends, rising demands, and opportunities to produce relevant pharmaceutical products. Top manufacturers are consistently evolving with time and technology, and they partake in industry consolidation.
Which supplier selection criteria are relevant for drug intermediates and API?
The majority of pharmaceutical giants outsource for APIs and drug intermediates for drug development. Given this, the competition among manufacturers is too high, and this can affect company evaluation and selection of the right suppliers. The wrong choice of suppliers can negatively influence the supply chain performance and overall performance of the pharmaceutical company.
Therefore, factoring supplier selection criterion before making purchases is essential. Some of the most important criteria for selection of API and drug intermediate suppliers include regulatory compliance (DMF status), cost, quality, supplier profile, risk, reliability, and financial profile of the supplier.
Key players of the pharmaceutical intermediates market
The pharmaceutical intermediates market comprises of many key players who have positively impacted the growth of the global market. Although some of these manufacturing companies independently produce pharma intermediates, a few others handle the production and development of intermediates as well as APIs.
Who are the key players in the global active pharmaceutical ingredients (APIs) market?
The global pharmaceutical market comprises a variety of pharma products in the pharmaceutical industry. APIs are one of the most prominent products, and several top manufacturing companies are key players in the active pharmaceutical ingredients market. Some of these key players include Pfizer Inc., Novartis International AG, Teva Pharmaceuticals, Aurobindo, Sun Pharmaceutical Industries Ltd., etc.
What factors have determined who the key players in the pharmaceutical intermediates market are?
Several factors have influenced and determined who the key players in the pharmaceutical intermediates market within different forecast period. These factors apply everywhere, regardless of the location of these top manufacturing companies. Factors that determine the key players include demographics or population science, technology, social behaviour and culture of the local community, politics (local, national and international), national and global regulating laws and policies, and the natural environment (raw material, pollution, energy prices, biotechnology), etc.
What are the differential strategies adopted by the key players to hold a significant share in the global pharmaceutical intermediates market?
Top manufactures of pharma intermediates have achieved their growth through the acquisition of smaller or other pharma – related companies and via mergers to establish their presence in the market. Also, top pharmaceutical intermediate companies are expanding their businesses through product portfolio expansion and development across the globe. Through these strategies, these key players can maintain a significant share in the global pharmaceutical intermediates market.
References
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
-
Webinar Leveraging Real-Time Clinical Data to Deliver Certainty in Solubility Enhancement and Modified Release Development
-
12th December 2024
-
3pm BST/ 4pm CET
-
-
Webinar The CPHI Sustainability Collective: A New Initiative to Support a Sustainable Pharma Value Chain
-
10th December 2024
-
3pm BST /4pm CET
-
-
Webinar Key to Success: A CDMO's Pathway to Biologics Excellence
-
5th November 2024
-
3pm BST/ 4pm CET
-
-
Webinar The Changing Dynamics of Global API Manufacturing
-
17th September 2024
-
3pm BST/ 4pm CET
-
-
Webinar Shaping the Future of Italy’s Pharma Market: Trends and Opportunities
-
4th September 2024
-
4pm CET/ 10am EST
-
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
-
Webinar The Next Frontier – Emerging Opportunities in the LATAM Pharma Market
-
21st November 2023
-
4pm CET / 10am EST
-
-
Webinar Vistamaxx™ MED - imagine the possibilities for healthcare product performance
-
10th October, 2023
-
4pm CET / 10am EST
-
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













-comp246169.jpg)


























































































































































































Grey-Overlay%20(1).jpg)
.jpg)
%20(2).jpg)
.jpg)
.jpg)

.jpg)







![Triphenylphosphine (TPP) [CAS# 603-35-0]](https://www.cphi-online.com/46/product/08/35/56/picture.jpeg)








![2-(3-Chloropropyl)-2, 5, 5-trimethyl -[1, 3]-dioxane](https://www.cphi-online.com/46/product/129/84/24/p0th_S.jpg)




![Fomepizole [7554-65-6]](https://www.cphi-online.com/46/product/124/54/03/p1245403th_S.jpg)






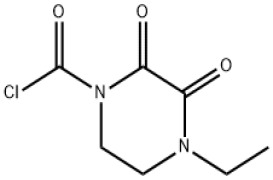





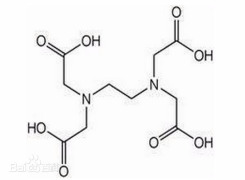



![sodium 2-{4,7,10-tris[2-(tert-butoxy)-2-oxoethyl]-1,4,7,10-tetraazacyclododecan-1-yl}acetate](https://www.cphi-online.com/46/product/128/93/59/p1289359th_S.jpg)





![1-[(tert-butoxy)carbonyl]azetidine-3-carboxylic acid](https://www.cphi-online.com/46/product/126/17/70/picture.png)


![(2S)-2-(8-amino-1-bromoimidazo[1,5-a]pyrazin-3-yl)-1-Pyrrolidinecarboxylic acid phenylmethyl ester](https://www.cphi-online.com/46/product/128/94/02/p0th_S.jpg)


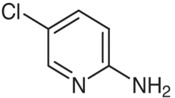





![2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyriMidine-6-carboxylic acid](https://www.cphi-online.com/46/product/68/69/17/p686917th_S.jpg)





![2-Hydroxy methyl 3-methyl-4-[2,2,2 trifluoroethoxy] pyridine hydrochloride](https://www.cphi-online.com/46/product/107/82/16/p1078216th_S.jpg)




































































.png)







.png)

.png)



.jpg)


