- Home
- Generic APIs
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products551,744
-
Companies7,781
-
Articles11,636
-
Events8
-
Webinars342
Generic APIs
- Generic Alkaloids
- Generic Amino Acids
- Generic Antibiotics
- Generic Antibodies
- Generic Antineoplastic / Chemotherape...
- Generic Carbohydrates
- Generic Cytokines
- Generic Enzymes
- Generic Hormones & Synthetic Substances
- Generic Peptides
- Generic Phospholipids
- Generic Probiotics
- Generic Prostaglandins
- Generic Sera and Vaccines
- Other Generic APIs
Generic APIs Companies (137)
Generic APIs News
-
News Scaling the Industry: CPHI Scale-Up Market interview with YSK Laboratories
For the first time, CPHI Milan hosted the CPHI Start-Up Market, expanding support for emerging and small-sized enterprises in their transition to the next level of growth. In this interview, we spoke with Yuvansh Khokhani, Managing Director of YSK Labo...19 Nov 2024 -
News Angels for Change: Guardians of the pharmaceutical supply chain
Angels for Change is an organisation that aims to build up resilience in the supply chain, by linking real life cases of patients that have been affected by drug shortages, and working to rectify and prevent them.2 May 2024 -
News Drug Patent Expiries: a steep cliff or opportunity for innovation?
The pharmaceutical industry faces a patent cliff together in the years leading up to 2030. Learn what this means for drug pricing, their outsourcing partners, and drug innovation of the future.30 Apr 2024 -
News Generics threat to Merck’s Bridion as Hikma seeks pre-patent expiry approval
Merck has disclosed they received notice from Hikma Pharmaceuticals for seeking a pre-patent expiry US FDA approval for Hikma’s generic version of Merck’s Bridion.28 Feb 2024 -
News Patients vs Pharma – who will the Inflation Reduction Act affect the most?
The Inflation Reduction Act brought in by the Biden administration in 2022 aims to give better and more equitable access to healthcare in the USA. However, pharma companies are now concerned about the other potential costs of such legislation.11 Dec 2023 -
News CPHI Barcelona Speaker Interview – What the US FDA’s Quality Management Maturity Means for the Pharma Industry
At CPHI Barcelona (24–26 October, 2023), we spoke to Sireesha Yadlapalli, CEO of Pharmatech Associates, who gave a presentation on the implications of the US FDA’s Quality Management Maturity (QMM) Initiative, and spoke on the panel of the ...1 Nov 2023 -
News UPDATE: CPHI Barcelona 2023 Innovator Interview – Hangzhou Huishi Consulting
Our Innovator Interviews takes a look at the cutting-edge strategies and technologies being implemented within the pharmaceutical supply chain through an in-depth discussion with a leading innovator in the industry.24 Oct 2023 -
News CPHI Podcast Series: Designing Drug Conjugates
Digital Editor Lucy Chard goes through the bioconjugate journey in this latest podcast from the CPHI Podcast Series, from development to manufacturing, with two experts in the field from Abzena, Louise Duffy and Campbell Bunce.29 May 2023 -
News Trend Report: The True Cost of API Price Rises
Download our Trend Report to discover what the current landscape of APIs and their pricing means for the pharmaceutical industry in the future.5 Dec 2022 -
News The Japanese Pharma Market – Top Four Trends Shaping the Sector
Drug pricing, generics, investment, innovation - how these trends are shaping the face of Japanese pharma20 Jul 2022 -
News Pfizer hit with patent infringement lawsuit over COVID-19 drug Paxlovid
A US-based biotech is seeking damages for Pfizer’s use of a patented coronavirus 3CL protease inhibitor in its blockbuster drug Paxlovid24 Jun 2022 -
News Boom expected in global COVID-19 drug associated APIs market
Market set to grow as companies face fewer operational restrictions and COVID-19 drug development continues at pace23 Mar 2022 -
News UPDATED: COVID-19 Vaccine and Therapeutic Development Tracker
The latest coronavirus updates and developments impacting the global pharmaceutical supply chain. Includes the latest news surrounding the therapeutic developments, updates to vaccines and boosters, and the global pharmaceutical regulations that need t...23 Aug 2024 -
News ANI Pharma enhances generics and CDMO business through Novitium acquisition
The transaction expands ANI’s R&D pipeline and adds nine new customers to its growing CDMO business24 Nov 2021 -
News Indian pharma industry on cusp of growth spurt, CPHI Worldwide audience told
Country expected to grow threefold over the next decade due to increased focus on complex generics, biosimilars and specialty products26 Oct 2021 -
News COVID-19 Pharma Development Tracker
The latest coronavirus updates and developments impacting the global pharmaceutical supply chain (continued from here)31 May 2021 -
News ANI Pharmaceuticals expands CDMO business through Novitium acquisition
The purchase will allow the company to add nine new customers to its growing CDMO business and 27 manufacturing suites10 Mar 2021 -
News CPHI Podcast Series: Digitalisation of API Supply Chains
Even before the COVID-19 pandemic, the pharmaceutical manufacturing sector was adopting digital technology to save time, cut costs and increase efficiencies in the value chain.18 Jan 2021 -
News CPHI Online Report - Pharma Market Trends 2021
Twelve pharma market trends we anticipate across 2021 for the drug development, manufacturing and outsourcing sectors.25 Nov 2020
Generic APIs Products (500+)
-
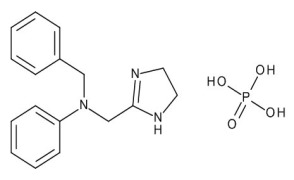
Product Amoxycillin trihydrate micronised
Amoxicillin trihydrate micronized, also sterile. Mixtures with potassium clavulanate and Syloid are available in various ratios.For more information please contact our sales team.
-
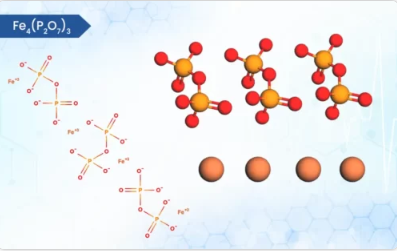
Product Ferric Pyrophosphate
Ferric pyrophosphate, a key iron source in some dietary supplements, boasts distinct physical and chemical properties. It exists as a yellowish-white to beige crystalline solid [1]. Unlike many iron compounds, it’s practically insoluble in water, rendering it odorless and tasteless [1]. Its white colour is...
-
Product API portfolio
Siegfried offers a broad portfolio of active pharmaceutical ingredients including controlled substances, focused on anesthetics, analgesics, decongestants, addiction treatment, and a variety of other therapeutic indications. In addition, we offer pharma grade caffeine for use in pharmaceutical and nutritio...
-
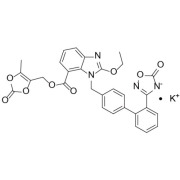
Product Ticagrelor
CTX Manufacturer Form - II
O DMF available.
CEP Approved.
CTX Lifesciences respects patent laws and conventions of pharmaceuticals as applicable in different countries.
API/Substances covered by patent are not offered to the countrie...
-
Product 1. Amifostine for Injection 500 mg 2. Azacitidine for Injection 100 mg 3. Bendamustine for Injection 25 mg and 100 mg 4. Bleomycin for Injection 15 Units and 30 Units 5. Bortezomib for Injection 1 mg, 2 mg, 2.5 mg and 3.5 mg 6. Busulfan Injection 60 mg/10
1. Amifostine for Injection 500 mg
2. Azacitidine for Injection 100 mg
3. Bendamustine for Injection 25 mg and 100 mg
4. Bleomycin for Injection 15 Units and 30 U...
-
Product ZLD, Zero Liquid Discharge
A Zero Liquid Discharge (ZLD) system is an advanced wastewater treatment process designed to eliminate liquid waste from a facility. By treating and recycling wastewater, ZLD systems recover water for reuse and concentrate the remaining waste into solid or liquid residues.
-
Product Rosuvastatin Ca
Status: Commercial; Specification: EP, USP, CP; Submission of Regulations: EDMF, CEP, USDMF, CDMF.
-
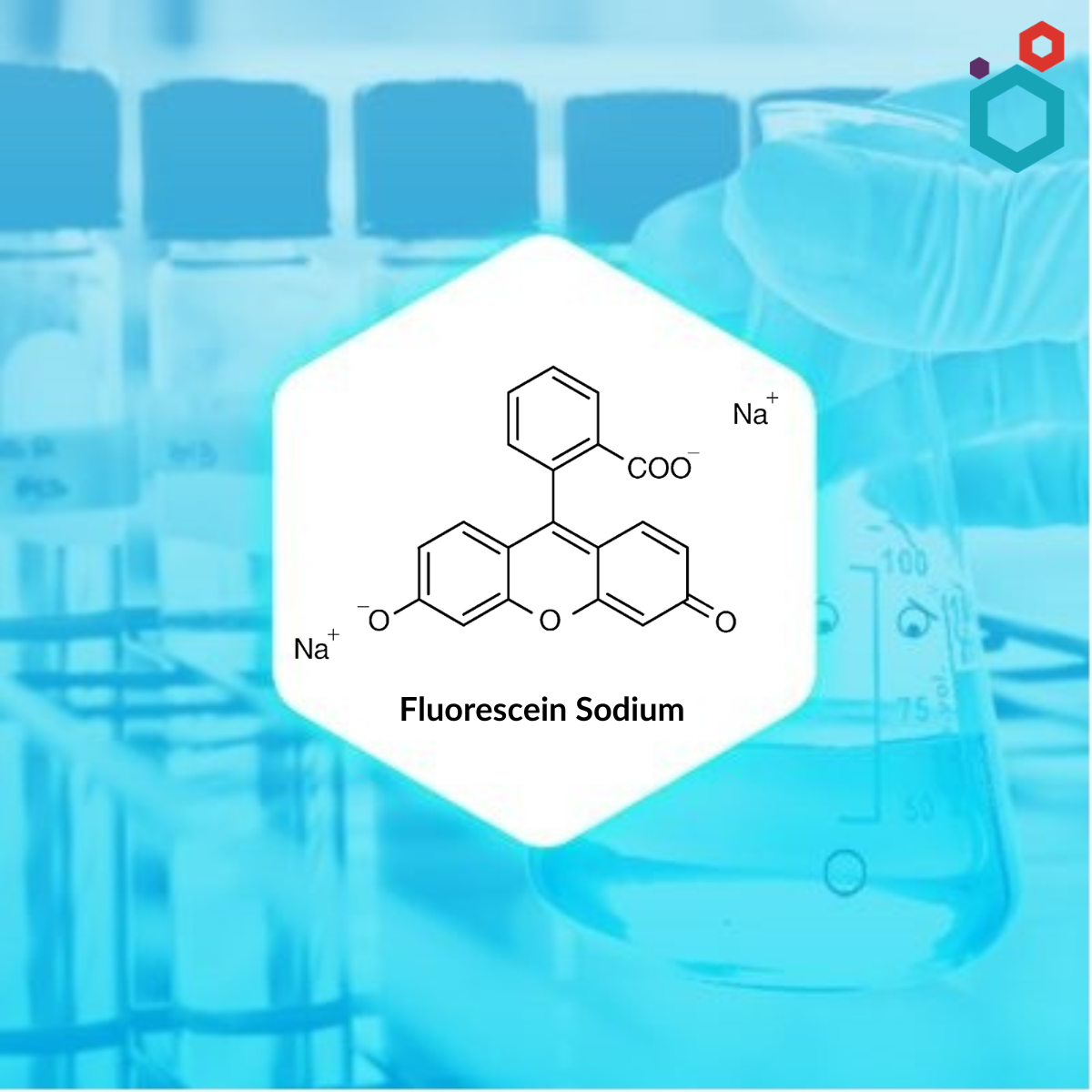
Product Fluorescein Sodium
Macsen laboratories offers a wide range of products which includes methylrosanilinium chloride bp. It belongs to Active pharmaceutical ingredients category. Appearance: dark green, shiny powder, hygroscopic. Solubility: sparingly soluble in water, freely soluble in ethanol (96%) and in methylene chloride. ...
-
Product API
Commercial :
• VONOPRAZAN FUMARATE • CLOPIDOGREL BISULFATE (Form II) • MEMANTINE HCL • VILDAGLIPTIN Under Plant Validations :
• PROMETHAZINE HCL • PROMETHAZINE BASE • MINOXIDIL • BILASTINE • MIRABEGRON • APIXABAN • RIVAROXABAN visit website for detailed product list : ht...
-
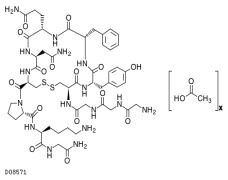
Product GENERIC PEPTIDES
BCNpeptides manufactures a wide range of Generic Peptides under strict GMP conditions at commercial scale for pharmaceutical and veterinary applications.
Our Generic Peptides are supported by regulatory filing in their respective markets and BCN Peptides will fully support you with the req...
-
Product Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5
Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5
N+1 in the base of Methotrexate API.
Quantity per batch about 30KG.
Monthly production capability 500KG.
Delivery time: stable stock, prompt delivery.
We supply series of Methotrexate products...
-
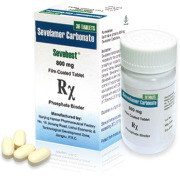
Product Sevelamer Carbonate API & Tablets
Sevelamer Carbonate API (CAS NO: 845273-93-0);
Sevelamer Carbonate Tablets is indicated for the control of serum phosphorus in patients with chronic kidney disease on dialysis.(Original company: Sanofi/ Genzyme, Original brand: Renvela/ Renagel)
-
Product Chlortetracycline HCL
Specification: EP/USP/BP/CHP/CVP Packing: 25Kgs/Drum or carton
Certifications: Chinese GMP, Chinese Veterinary Drug GMP, U.S.FDA, EU-GMP, COS, MAFF approval
Product Advantages:
●Uniquely approved for human use grade
●Fine solubility
●Fine color
●High Biological Assay

-
Product Amitriptyline Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Amitriptyline Hydrochloride. It belongs to commercial products category. Therapeutic category: Antidepressant. Contact us for more information.
-
Product API Brokering - Small Molecule
Savendor offers API brokerage services to connect you with our 160+ API manufacturer partners who make over 2500 different APIs. Our list of partners is constantly growing and we can access items that are not listed on our website.
Use our escrow system at no cost when purchasing through Savendor a...
-
Product PRAMIPEXOLE DIHYDROCHLORIDE MONOHYDRATE
Situated on the island of Malta, just south of Italy in the Mediterranean Sea, Amino Chemicals was founded in 1992, and is part of ABA Chemicals Corporation. Following consecutive GMP audits by both FDA and MMA (EMA’s competent authority in Malta), it is focused on the chemical process development and...
-
Product Active Pharmaceutical Ingredients (APIs)
We provide the high quality active pharmaceutical ingredients globally. We are one of the leading manufacturers of active pharmaceutical ingredients in the world.
Balsalazide Disodium Dihydrate (US, TW, IN, KR)
Benzonatate (US, TW, IN, MX, CN, SG)
Calcipotriol Anhydrous ...
-
Product KSM & API Solutions
We supply high-quality Active Pharmaceutical Ingredients (APIs), adhering to cGMP standards. Our approach is innovative, starting with paperless labs for efficient R&D. Quality by Design principles guide our process design, ensuring safety and efficiency.
Our technology transfer ensures a se...
-
Product Generic APIs
Grace's Fine Chemical Manufacturing Services (FCMS) has a long history of supplying high-quality generic and custom APIs to the pharmaceutical and nutraceutical markets. Grace's FCMS has manufactured some of the most notable APIs that are now part of our portfolio of generic products available to devel...
-
Product CordenPharma Small Molecules Platform
Full-Service Partner of Choice from Advanced Intermediates to Drug Products • Integrated network of cGMP facilities across Europe and US • 1,200 m3 of volumetric capacity in 20 L - 28,000 L reactors with various materials of construction • Clinical (Phase I-III) and ongoing commercial supply to multiple ...
-
Product Venetoclax
We offer an integral secure solution as your startegic partner of Venetoclax.
Venetoclax (CAS 1257044-40-8) is a targeted therapy drug used to treat chronic lymphocytic leukaemia (CLL), manufactured in our unique European GMP manufacturing plant located in Barcel...
-
Product Sodium Polystyrene Sulfonate USP/EP (Doshion P 504)
DOSHION P 504 is Sodium Polystyrene Sulfonate, a strong acid cation exchange resin powder. This is a pharmaceutical grade product which is used for the treatment of a specific medical condition.In certain cases there can be a build up of potassium in the bloodstream which is not removed by the kidneys...
-
Product Pantoloc Tablet
Active ingredient: Pantoprazole ( as Pantoprazole Sodium Sesquihydrate)
Indication: Treatment of reflux oesophagitis, gastric & duodenal ulcers, Zollinger - Ellison Syndrome
Forms: Pantoloc 20 mg Tablet
&n...
-
Product Solid State Research & Development Services
Eurofins CDMO provides expertise and services in physicochemical characterization of API and intermediates, screening for different API solid forms and salts as well as crystallization process development.
We apply High-Throughput Screening Technology (HTST) for expeditious discovery of new poly...
-
Product Semaglutide
Semaglutide is an analog of glucagon-like peptide-1 (GLP-1). It is indicated as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes mellitus. And also, to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established c...
-
Product Terlipressin Acetate
Analogue of vasopressin used as a vasoactive drug in the management of low blood pressure. It has been found to be effective when norepinephrine does not help.
-
Product OFF-PATENT APIs
High value applications Hovione provides off-patent APIs that can be used with confidence where the choice of active ingredient is critical.
• Branded generics where quality and security of supply is paramount • Difficult to make AB rated generics such as nasal and inhalation products where devel...
-
Product Prednisolone base/acetate
We are very compeitive on Prednisolone base/acetate.Our mfr is GMP certified for this item with WC/CEP document.
-
Product API-Dextran 60
roduct name: Dextran 60
Normative Mw: 60000
CAS NO: 9004-54-0
Appearance: White Crystal powder
Specification: BP, EP, USP, CP
Documentation: GMP, DMF, EU written confirmation
Application: Blood Volume Expander, Organ Preservation, V...
-
Product Contract Manufacturing Solutions
GVK BIO offers long term Contract Manufacturing Solutions in development, validations, DMF filing, manufacturing of New Chemical Entities (NCE), Active Pharmaceutical Ingredients (API) and Intermediates.
Capabilities:Environment friendly routes under GMP conditions
Robust and cost-effective p...
-
Product Purolite® C100CaMRNS
Calcium Polystyrene Sulfonate active pharmaceutical ingredient for potassium control supplied in the powder form. The product is produced by chemical synthesis and is composed of styrene crosslinked with divinylbenzene, sulfonic acid in the calcium form. This product has a Drug Master File registered in Eu...
-
Product Generic APIs
Creating high-quality, affordable active pharmaceutical ingredients (APIs) is one of the keys to help us bring good health to all. Our API Business caters to leading innovator and generic companies across the US, Europe, Latin America, Japan, Korea and other emerging markets. Over the years, we have develo...
-
Product QUETIAPINE FUMARATE API
Medichem S.A. offers wide range of pharmaceutical APIs which includes Quetiapine Fumarate. Indication: Depressive episodes associated with bipolar disorder. Contact us for more information.
-
Product Abarelix
Product name:AbarelixMolecular Formula.: C72H95ClN14O14 Molecular Weight.: 1416.10 Fields of Application: Advanced symptomatic prostate cancer Active Substance:Abarelix is an injectable gonadotropin-releasing hor...
-
Product Mapbiopharma
Specialized in the supply of raw materials and finished products for the pharmaceutical, veterinary and nutritional industry around the world. We have our own laboratory "Netpharmalab" in addition to manufacturing dermatological finished products, food supplements and bio-sanitary products.
-
Product Povidone Iodine
Jiaozuo zhongwei special products pharmaceutical co. Ltd offers a wide range of product which includes povidone iodine. Properties: exists as free flowing,redish brown powder,non-irritant with good stability,dissolve in water and alcohol,insoluble in diethylethe and chloroform. Applications: it is one of t...
-
Product Product list of API
Tamoxifen Citrate
Duloxetine Hydrochloride
Clopidogrel Bisulfate
Clozapine
Pantoprazole Sodium
Omeprazole
Lansoprazole
Sulfamethoxazole
Metformin HCl
Hydroxychloroquine sulfate
Cilostazol
Trospium Chloride
Hydrocortisone q...
-
Product Gmp ethanol
Kimia offers a wide range of products which includes GMP ethanol. Uses and applications: it is a natural solvent meeting GMP requirements for use in pharmaceutical applications. Contact us for more information.
-
Product Polyethylene Glycol 3350 API / Polyethylene Glycol 4000 API
By precise polymerization technology and advanced refining post-processing technology to ensure batch-to-batch repeatability.
Spray granulation process gives good flowability, and the particle size can be customized according to the requirements of clients.
The product quality meets the domestic and ...
-
Product Sevoflurane API, Propofol API and Glycofurol excipient
High quality APIs, Minimum impurities offered by Troikaa Pharmachem Pvt. Ltd.; a wholly owned subsidiary of Troikaa Pharmeceuticals Ltd. • Sevoflurane • Propofol • Remifentanyl • Ketamin • Dexmedetomidine • Midazolam • Rocuronium Bromide • Dequalinium • Glycofurol (Excipient)
-
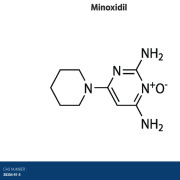
Product Minoxidil
Flamma Spa offers minoxidil so please reach out to learn more directly via FlammaGroup.com
-
Product Mupirocin base
Topical antibiotic. It binds reversibly to isoleucine transfer RNA synthetase, preventing isoleucine from entering the cell, thereby stopping the synthesis of all isoleucine-containing proteins in the cell. It is used for acute primary bacterial skin infections, such as folliculitis and impetigo.
-
Product Phloroglucinol
Hepartex distributes a wide range of APIs which includes Phloroglucinol. Please contact us for more information.
-
Product Azithromycin
(2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one. The degree of hydration is1 or 2.Semi-synthetic...
-
Product Butorphanol tartrate
Butorphanol tartrate is a synthetic opioid analgesic and is used for the management of moderate to severe pain, as well as for sedation in certain medical procedures.
It is commonly used in both human and veterinary medicine. In veterinary medicine, it’s often used for pre-anesthetic medicat...
-
Product Semaglutide
Hybio Pharmaceutical Co., Ltd. offers a wide range of pharmaceutical products which includes calcitonin(salmon). It belongs to active pharmaceutical ingredient category. Contact us for more information.
-
Product Terizidone
Enzychem lifesciences Corporation offers a wide range of products and services.Contact us for more information.
-
Product GUAIFENESIN
Guaifenesin is an expectorant. It helps loosen congestion in your chest and throat, making it easier to cough out through your mouth.
-
Product Solid forms Manufacturing
Our solid forms plant exports 75% of its output, supplying products to 45markets in 25 different languages.One of the biggest FDA-approved plant for solid forms in Europe with an annual capacity of 3 billion tablets. We use the latest technology to produce high-quality products ata very competitive Price.L...
-
Product Metaraminol Bitartrate
Metaraminol bitartrate is a potent sympathomimetic amine that increases both systolic and diastolic blood pressure.

-
Product Oncology Injectables, Oral Solid Dosage Forms & APIs
Quality products manufactured in highly compliant facility for registration and sales in highly regulated markets.
-
Product Inhalation APIs
ARFORMOTEROL, FORMOTEROL, GLYCOPYRROLATE, INDACATEROL, OLODATEROL SALMETEROL, TIOTROPIUM, UMECLIDINIUM and VILANTEROL

-
Product Zent2U - Zentiva B2B Platform
Zent2U (www.zent2u.com) is a B2B platform of Zentiva established in 2019 to collaborate with customers on:
- Licensing-out dossiers developed by Zentiva
- Co-development opportunities by co-financing Zentiva's products in development
- CMO services by Zentiva in production ...
-
Product Teriparatie
Chunghwa chem synthesis & biotech co. offers a wide range of api products which includes Teriparatie. It is labeled for the treatment of osteoporosis. Contact us for more information.
-
Product Mefenamic Acid (USDMF, CEP, Canada-DMF)
USP/BP/EP/JPGMP certificate, 2018 EIR cover letter USDMF and CEP available Supply worldwide
-
Product Rosuvastatin + Fenofibrate
Ravenbhel Healthcare Pvt. Ltd. Offers a wide range of products which includes Rosuvastatin + Fenofibrate
-
Product Candesartan cilexitil
Candesartan is an angiotensin II receptor antagonist used mainly for the treatment of hypertension.
-
Product Addera D3
HYPERMARCAS S.A Offers A Wide Range Of Over-The-Counter Medications Which Includes Addera D3. Active Ingredients: Colecalciferol (Vitamin D3) 3300 UI / Ml. Contact us for more information.
-
Product Guanine
In the cosmetics industry, crystalline guanine is used as an additive to various products (e.g., shampoos), where it provides a pearly iridescent effect. It is also used in metallic paints and simulated pearls and plastics. It provides shimmering luster to eye shadow and nail ...
-
Product Potassium clavulanate
Potassium clavulanate in mixture with AMoxycillin trihydrate. Also micronised, and sterile.
-
Product Permethrin 25/75 Ph.Eur.
Alchimica offers a wide range of insecticide active ingredients for cosmetic and pharmaceutical use.
-
Product API
Alchimica offers a wide range of active pharmaceutical ingredients for human and veterinary use. More information can be found in our Pharmaceuticals brochure.
-
Product Liposomal Iron
Liposomal iron isn’t a single, well-defined compound but rather a delivery system for iron. It encapsulates iron molecules within microscopic spheres made of phospholipids, the same fatty molecules that form cell membranes. These spheres, called liposomes, are typically colorless to slightly yellow and ran...
-
Product Iron Sucrose
Iron sucrose is a complex molecule used to replenish iron stores in individuals with deficiency, particularly those with chronic kidney disease. Unlike oral iron supplements, iron sucrose is administered intravenously. Physically, it appears as a brown to reddish-brown sterile solution and is odorless. Int...
-
Product Liposomal Iron, Liposomal Calcium, Liposomal Magnesium, Liposomal Zinc, Liposomal Vitamin C, Liposomal ALA, Liposomal Glutathione, Liposomal Curcumin, Liposomal Vitamin B12, Liposomal Vitamin K2, Liposomal Thiamin
Liposomal Iron - It encapsulates iron molecules within microscopic spheres made of phospholipids, the same fatty molecules that form cell membranes. Liposomal Calcium - It is the encapsulation of calcium ions within a liposome structure for the potential delivery of calcium ions across the cell m...
-
Product Liposomal Iron, Ferrous Bisglycinate, Ferric Citrate, Iron Polymaltose, Sucroferric Oxyhydroxide, Iron Isomaltoside, Ferric Derisomaltose,Ferric Pyrophosphate, Ferric Carboxymaltose,Ferric Ammonium Citrate,Ferrous Ascorbate,Ferric Maltol.
WBCIL is a leading manufacturer of Iron supplementations which are in the form of chelated iron,Iron complex, Liposomal Iron & Injetable iron. The products qualities are confirmed by different characteristics analysis like FTIR,NMR,XRD,Mass spectroscopy & many more. Many o...
-
Product L-Methylfolate ,Boron Citrate, Boron Glycinate, Sucroferric Oxyhydroxide, Enclomiphene Citrate , Sodium Butyrate Food / USP, Calcium Butyrate Food Grade , Ulipristal Acetate, Ascorbyl Palmitate, Dimethyl Fumarate, L Ornithine L Aspartate,
L-Methylfolate Boron Citrate
Boron Glycinate
Sucroferric Oxyhydroxide
Enclomiphene Citrate
Sodium Butyrate Food / USP
Calcium Butyrate Food Grade
Ulipristal Acetate
Ascorbyl Palmitate
Dimethyl Fumarate
L Ornithine L Aspartate
-
Product Calcium Lactate Gluconate,Calcium Levulinate,Calcium Pidolate,Calcium Butyrate,Coral Calcium, Calcium Gluconate,Calcium Aspartate,Calcium Orotate,Calcium Bisglycinate,Calcium Acetate, Liposomal Calcium,Calcium Citrate,Calcium Citrate Malate, Milk Calcium
Calcium is essential for bone health, muscle function, and cellular activity. West Bengal Chemical Industries Limited (WBCIL) offers an extensive range of high-quality calcium compounds to meet diverse industry needs. Our portfolio includes Calcium Gluconate for deficiency treatment, Calcium Lactate G...
-
Product ZINC Acetate,ZINC Citrate,ZINC Bisglycinate,ZINC Gluconate,MAGNESIUM Acetate,MAGNESIUM Citrate,MAGNESIUM Glycinate,POTASSIUM Acetate,POTASSIUM Propionate,POTASSIUM Glycinate,POTASSIUM Orotate,COPPER Acetate,COPPER Citrate, COPPER Glycinate
ZINC - Liposomal, Acetate, Propionate, Citrate, Ascorbate, Aspartate, Bisglycinate, GluconateMAGNESIUM- Liposomal, Acetate, Propionate, Citrate,Ascorbate, Aspartate, Bisglycinate, Gluconate
POTASSIUM - Acetate, Propionate , Citrate› Glycinate , Orotate, Mag. Citrate, Aspartate qq...
-
Product 1. Amifostine for Injection 500 mg
1. Amifostine for Injection 500 mg
2. Azacitidine for Injection 100 mg
3. Bendamustine for Injection 25 mg and 100 mg
4. Bleomycin for Injection 15 Units and 30 U...
-
Product Linagliptin
Linagliptin is in a class of medications called dipeptidyl peptidase-4 (DPP-4) inhibitors.
-
Product Solvent stripping unit
A tailor-made solvent stripping unit is an industrial system precisely customized to meet the specific requirements of a given process or product. Engineered to efficiently remove residual solvents from liquid mixtures, it is designed with a focus on minimizing operational costs.
-
Product Aerobic system
These secondary treatments are used to break down any biodegradable organic matter and to remove non-settleable solids that cannot be separated by physical treatment.
-
Product Chemical-physical plant
The chemical-physical treatment allows the processing of heavily polluted wastewater, including those with suspended metals, which cannot be treated using biological systems. These wastewater treatments utilize the chemical processes of flocculation and sedimentation, combined with the physical pr...
-
Product Atorvastatin Ca
Status: Commercial; Specification: EP,USP,CP;Submission of Regulations: EDMF, CEP, USDMF under preparing, CDMF.
-
Product Valsartan
Status: Commercial; Specification: EP,USP;Submission of Regulations:EDMF, CEP, USDMF, CDMF.
-
Product Losartan Potassium
Status: Commercial; Specification: EP, CP; Submission of Regulations: EDMF, CEP, USDMF, CDMF
-
Product Pregabalin
Status: Commercial; Specification: EP, CP; Submission of Regulations:CEP under preparing, CDMF
-
Product Calcium Levofolinate GMP Manufactuere CAS NO.: 80433-71-2
Calcium Levofolinate GMP Manufactuere CAS NO.: 80433-71-2
Quantity per batch about 17KG.Monthly production capability 200KG.
Delivery time: Production against order.
We supply series of Calcium Levofolinate products: Methotrexate, Methotrexate sodium, Calcium folina...
-
Product L-5-Methytetrahydrofolate Calcium GMP Factory Cas No.151533-22-1
L-5-Methytetrahydrofolate Calcium GMP Factory Cas No.151533-22-1
Specifications: Enterprise standards.
We supply series of L-5-Methytetrahydrofolate Calcium products: Methotrexate, Methotrexate sodium, Calcium folinate(Leucovorin calcium), Calcium Levofolinate.
...
-
Product Methotrexate GMP manufacturer Cas No.59-05-2
• Methotrexate GMP manufacturer Cas No.59-05-2
Available documents: GMP, DMF, WC, COPP, DML.
Quantity per batch about 30KG.
Monthly production capability 500KG.
Delivery time: Top one product, stable stock, prompt delivery.
We supply series of Meth...
-
Product Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5
Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5
N+1 in the base of Methotrexate API.
Quantity per batch about 30KG.
Monthly production capability 500KG.
Delivery time: stable stock, prompt delivery.
We supply series of Methotrexate products...
-
Product Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5
Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5
N+1 in the base of Methotrexate API.
Quantity per batch about 30KG.
Monthly production capability 500KG.
Delivery time: stable stock, prompt delivery.
We supply series of Methotrexate products...
-
Product Calcium Folinate (Leucovorin Calcium) GMP Certificate CAS No.:1492-18-8
Calcium Folinate (Leucovorin Calcium) GMP Certificate CAS No.:1492-18-8Specifications: CP, EP, USP.
Available documents: GMP, DMF, WC, COPP, DML.
Quantity per batch about 50KG.
Monthly production capability 500KG.
Delivery time: Top one product, stable stock, prompt...
-
Product Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5
Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5N+1 in the base of Methotrexate API.
Quantity per batch about 30KG.
Monthly production capability 500KG.
Delivery time: stable stock, prompt delivery.
We supply series of Methotrexate products: Methotrexate, Methotrexate sodium, Calcium...
-
Product Iron Sucrose API & Ferrofer(iron sucrose injection)
Iron Sucrose API: CAS No.: 8047-67-4
Ferrofer (Iron Sucrose Injection) is indicated for parenteral treatment of iron deficiency in cases whereby oral iron preparations cannot provide for sufficient supplementation.

-
Product Gentamicin sulphate
Specifications: EP/USP/JP/CHP
Packing: 5KB/Tin, 2Tins/carton
Certifications: Chinese GMP, U.S.FDA, EU-GMP, COS, PMDA approval
Product Advantages:
●Fine color
●Low impurity level
●Low bacterial endotoxin
●High Biological ...
-
Product TETRACYCLINE HYDROCHLORIDE
Specification: EP/USP/BP/IP Packing: 25Kgs/ Carton
Certifications: Manufacturing license, EU-GMP,Indian DCGI approval,Pending approval by U.S.FDA, COS application in progress.
Product Advantages:
●High productivity
●Fine solubility
●Fine color
●High Biological As...
-
Product Cefmetazole Sodium Sterile
Specification: CHP/JP/USP Certification: Taiwan's DMF,Chinese GMP,PMDA approval,KFDA Approval
-
Product Bupropion Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Bupropion Hydrochloride. It belongs to commercial products category. Therapeutic category: Antidepressant. Contact us for more information.
-
Product Cyclobenzaprine Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Cyclobenzaprine Hydrochloride. It belongs to commercial products category. Therapeutic category: Muscle Relaxant. Contact us for more information.
-
Product Diclofenac Potassium
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Diclofenac Potassium. It belongs to commercial products category. Therapeutic category: Anti-inflammatory. Contact us for more information.
-
Product Diclofenac Sodium
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Diclofenac Sodium. It belongs to commercial products category. Therapeutic category: Anti-inflammatory. Contact us for more information.
-
Product Dothiepin Hydrochloride (Dosulepin HCl)
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Dothiepin Hydrochloride (Dosulepin HCl). It belongs to commercial products category. Therapeutic category: Antidepressant. Contact us for more information.
-
Product Doxepin Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Doxepin Hydrochloride. It belongs to commercial products category. Therapeutic category: Antidepressant.Contact us for more information.
-
Product Entacapone
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Entacapone. It belongs to commercial products category. Therapeutic category: Antiparkinsonian. Contact us for more information.
-
Product Fexofenadine Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Fexofenadine Hydrochloride. It belongs to commercial products category. Therapeutic category: Antihistaminic. Contact us for more information.
-
Product Glibenclamide (Glyburide)
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Glibenclamide (Glyburide). It belongs to commercial products category. Therapeutic category: Antidiabetic. Contact us for more information.
-
Product Ibuprofen Lysine Salt
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Ibuprofen Lysine Salt. It belongs to commercial products category. Therapeutic category: Analgesic. Contact us for more information.
-
Product Isosorbide Dinitrate
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Isosorbide Dinitrate. It belongs to commercial products category. Therapeutic category: Vasodilator. Contact us for more information.
-
Product Isosorbide-5-Mononitrate
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Isosorbide-5-Mononitrate. It belongs to commercial products category. Therapeutic category: Vasodilator. Contact us for more information.
-
Product Modafinil
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Modafinil. It belongs to commercial products category. Therapeutic category: Psychostimulant. Contact us for more information.
-
Product Nifedipine
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Nifedipine. It belongs to commercial products category. Therapeutic category: Antihypertensive. Contact us for more information.
-
Product Nitroglycerine (Glyceryl Trinitrate)
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Nitroglycerine (Glyceryl Trinitrate). It belongs to commercial products category. Therapeutic category: Vasodilator. Contact us for more information.
-
Product Nortriptyline Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Nortriptyline Hydrochloride. It belongs to commercial products category. Therapeutic category: Antidepressant. Contact us for more information.
-
Product Piroxicam
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Piroxicam. It belongs to commercial products category. Therapeutic category: Anti-inflammatory. Contact us for more information.
-
Product Selegiline Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Selegiline Hydrochloride. It belongs to commercial products category. Therapeutic category: Antiparkinsonian. Contact us for more information.
-
Product Tolbutamide
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Tolbutamide. It belongs to commercial products category. Therapeutic category: Antidiabetic. Contact us for more information.
-
Product Triamterene
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Triamterene. It belongs to commercial products category. Therapeutic category: Diuretic. Contact us for more information.
-
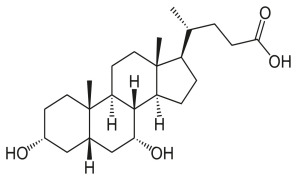
Product Ursodeoxycholic Acid
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Ursodeoxycholic Acid. It belongs to commercial products category. Therapeutic category: Cholelitholytic Agent. Contact us for more information.
-
Product Zonisamide
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Zonisamide. It belongs to commercial products category. Therapeutic category: Anticonvulsant. Contact us for more information.
-
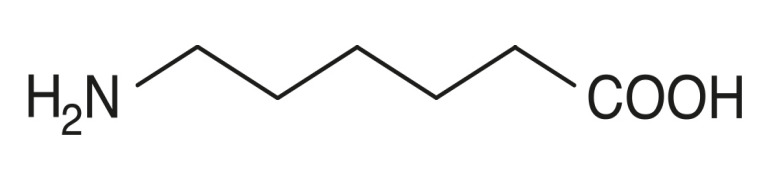
Product Aminocaproic Acid
Dipharma manufactures wide range of Active Pharmaceutical Ingredients which includes Aminocaproic Acid. It belongs to new products category. Therapeutic category: Treatment of bleeding disorders. Contact us for more information.

-
Product Chenodeoxycholic Acid*
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Chenodeoxycholic Acid*. It belongs to new products category. Therapeutic category: Bile Stone Therapy, Plain antitussives. Contact us for more information.
* only for specific markets. See our web site
-
Product Crisaborole
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Crisaborole. It belongs to new products category. Therapeutic category: Treatment of atopic dermatitis and psoriasis. Contact us for more information.
-
Product Dapsone
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Dapsone. It belongs to new products category. Therapeutic category: Antibacterial. Contact us for more information.
-
Product Dexlansoprazole Anhydrous Crystalline
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Dexlansoprazole Anhydrous Crystalline. It belongs to new products category. Therapeutic category: Antiulcerative. Contact us for more information.
-
Product Efinaconazole
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Efinaconazole. It belongs to new products category. Therapeutic category: Antifungal, Treatment of onychomycosis. Contact us for more information.
-
Product Fesoterodine Fumarate
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Fesoterodine Fumarat. It belongs to new products category. Therapeutic category: Antimuscarinic, Treatment of urinary incontinence. Contact us for more information.
-
Product Minodronic Acid
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Minodronic Acid. It belongs to new products category. Therapeutic category: Treatment of osteoporosis. Contact us for more information.
-
Product Pirfenidone
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Pirfenidone. It belongs to new products category. Therapeutic category: Immunosuppressant. Contact us for more information.
-
Product Rufinamide
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Rufinamide. It belongs to new products category.
Therapeutic category: Anticonvulsant.
Contact us for more information.
-
Product Selegiline Base*
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Selegiline Base*. It belongs to new products category. Therapeutic category: Antidepressant, Antiparkinson. Contact us for more information.
* only for specific markets. See our website
-
Product Tauroursodeoxycholic Acid
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Tauroursodeoxycholic Acid. It belongs to new products category. Therapeutic category: Bile Stone Therapy. Contact us for more information.
-
Product Vortioxetine HBr
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Vortioxetine HBr. It belongs to new products category. Therapeutic category: Antidepressant. Contact us for more information.
-
Product Benserazide Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Benserazide Hydrochloride. It belongs to new products category. Therapeutic category: Antiparkinson. Contact us for more information.
-
Product Biperiden Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Biperiden Hydrochloride. It belongs to new products category. Therapeutic category: Antiparkinson. Contact us for more information.
-
Product Varenicline Tartrate
Dipharma manufactures wide range of active pharmaceutical ingredients including Varenicline Tartrate, a smoking-cessation product, which is nitrosamine free due to the dedicated process developed. Contact us for more information.

-
Product Upadacitinib
Situated on the island of Malta, just south of Italy in the Mediterranean Sea, Amino Chemicals was founded in 1992, and is part of ABA Chemicals Corporation. Following consecutive GMP audits by both FDA and MMA (EMA’s competent authority in Malta), it is focused on the chemical process development and...
-
Product Brivaracetam
Situated on the island of Malta, just south of Italy in the Mediterranean Sea, Amino Chemicals was founded in 1992, and is part of ABA Chemicals Corporation. Following consecutive GMP audits by both FDA and MMA (EMA’s competent authority in Malta), it is focused on the chemical process development and...
-
Product Amisulpride
LEBSA manufactures in compliance with full GMP conditions a range of Active Pharmaceutical Ingredients (APIs) which includes Amisulpride (CAS number 71675-85-9). It is a neuroleptic/ antipsychotic agent manufactured in our unique European GMP manufacturing plant ...
-
Product Betahistine dihydrochloride
Our product Betahistine dihydrochloride (CAS number 5579-84-0) is an antivertigo agent manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Our active ingredient Betahistine dihydrochloride (CAS number 5579-84-0) is ...
-
Product Dequalinium chloride
Our product Dequalinium chloride (CAS number 522-51-0) is an Antiseptic agent manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Our active ingredient Dequalinium chloride (CAS number 522-51-0) is available with f...
-
Product Betahistine Dimesilate
Our product Betahistine Dimesilate/ Mesilate (CAS number 54856-23-4) is an antivertigo agent manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Our active ingredient Betahistine Dimesilate/ Mesilate (CAS number 54856-23...
-
Product Bromopride
Our product Bromopride (CAS number 4093-35-0) is an antiemetic agent manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Our active ingredient Bromopride (CAS number 4093-35-0) is available with full regulatory support, ...
-
Product Histamine dihydrochloride
Our product Histamine dihydrochloride (CAS number 56-92-8) is an antineoplastic agent and diagnostic aid (skin prick testing) manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Our active ingredient Histamine dihydrochloride (CAS...
-
Product Picloxydine dihydrochloride
Our product Picloxydine dihydrochloride (CAS number 19803-62-4) is an antiseptic agent manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Our active ingredient Picloxydine dihydrochloride (CAS number 19803-62-4)&nb...
-
Product Dibrompropamidine isetionate
Our product Dibrompropamidine isetionate (CAS number 614-87-9) is an antiseptic agent manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Our active ingredient Dibrompropamidine isetionate (CAS number 614-87-9) ...
-
Product Propamidine isetionate
Our product Propamidine isetionate (CAS number 140-63-6) is an antiseptic agent manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Our active ingredient Propamidine isetionate (CAS number 140-63-6) is availab...
-
Product Lacidipine
Our product Lacidipine (CAS number 103890-78-4) is an antihypertensive agent (calcium channel blocker) manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Our active ingredient Lacidipine (CAS number 103890-78-4) is...
-
Product Octenidine dihydrochloride
Octenidine dihydrochloride (CAS 70775-75-6) is an antiseptic manufactured in our unique European GMP manufacturing plant located in Barcelona, Spain. Octenidine dihydrochloride (CAS 70775-75-6) is available with full regulatory support, inc...
-
Product Tiapride hydrochloride
LEBSA manufactures in compliance with full EU GMP conditions a range of Active Pharmaceutical Ingredients (APIs) which includes Tiapride hydrochloride (CAS number 51012-33-0). It is a neuroleptic/ antipsychotic agent manufactured in our unique European GMP manufacturing&n...
-
Product Mianserin
Mianserin (CAS number 24219-97-4) is a antidepressant agent . Mianserin is available with full regulatory support, including CEP (submitted 03/2021)/ EU DMF/ GMP certificate. LEBSA is your reputed API manufacturer partner and well known for the quality of the produc...
What are Generic APIs?
Generic APIs (Active Pharmaceutical Ingredients) contain the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is believed to be equivalent in performance.A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging.
Search for Generic API suppliers online from our list of over 750 generic products from over 150+ suppliers located across the globe.
Generic Active Pharmaceutical Ingredients (APIs): Importance and Market Trends
Every drug you’ll come across is made using two core components – an Active Pharmaceutical Ingredient (API), also referred to as the central ingredient, and an excipient, which is simply a chemically inactive substance within the drug that helps carry the medication into your system. While an excipient can be any common substance, such as mineral oil or lactose, the APIs are what make the medication exhibit its fundamental properties.
This article highlights the importance of APIs to patients, their development and importance, and the current market trends concerning their manufacture and supply.
What are Generic Active Pharmaceutical Ingredients (APIs)?
Simply put, Active Pharmaceutical Ingredients (APIs) are the part of a drug that produces its intended effects. They are mostly synthetic substances/chemicals that are used in a carefully measured and formulated final pharmaceutical dosage. Certain drugs, such as those used in combination therapies, may have more than one active ingredient to treat various symptoms of an illness, and might also act in different ways.
APIs are often confused with the term ‘raw material’ and are sometimes also used as synonyms. However, there is a difference between the two. Raw materials are the chemical compounds which serve as the base from which APIs are made. This mechanism does not involve a single reaction; rather raw materials undergo a series of chemical processes and intermediates after which a purified and refined API is developed.
Where are Generic APIs Developed?
APIs are traditionally manufactuered by the pharmaceutical companies locally. However, recent years have seen a shift, where many corporations have outsourced their manufacturing processes to overseas companies in efforts to reduce the costs. Despite being an economical decision, this has resulted in major changes as to how drugs are regulated, giving rise to more stringent guidelines and inspections.
What is the Importance of Generic APIs to Patients and Pharmacists?
First off, generic APIs prevent drugs from being mistaken for one another, especially when prescriptions are distributed. To make sure there is no confusion and fatal error, the US Food and Drug Administration (FDA) must approve every proposed brand name or non-generic API for a drug, which will be discussed in detail in the upcoming section.
Secondly, government officers, healthcare workers, researchers, and those writing about a new active compound always use the drug’s generic name, since it refers exclusively to the drug, not to any particular brand or a specific product.
Thirdly, generic APIs help pinpoint the patient and vendor to the particular drug that is required. Sometimes a particular brand’s medicine is not available, in which case the pharmacist will enquire the generic API and provide the patient an alternate brand containing the same active ingredient.
How Do Generic APIs Differ from Non-Generic APIs?
Generic APIs refer to medications being sold using the name of the active ingredient, also known as generic drugs. Non-generic APIs refers to medications that use the same active ingredient, or APIs, as the original generic medication, but sell the drug using a brand-name instead of the name of the API.
Simply stated, the brand name, or non-generic API, is the name the company gives to their drug, and is generally easy to understand and use by common people for sales and marketing. The generic name, or generic API, is the name of the active ingredient contained within the drug.
In the US, the generic name is allocated by an official body known as the United States Adopted Names (USAN) Council, whereas the brand name is devised by the company asking for approval of the drug, the latter being their exclusive.
For instance, a common anti-seizure drug is phenytoin, which is the generic name (API) and Dilantin is the brand name for the same drug.
Brand-name drugs can only be manufactured and sold by companies that hold the patent for the drug, and these can be bought over-the-counter with a prescription. After the patent expires, a generic version of the brand-name drug can be manufactured and sold. These drugs cost between 30 to 95 percent less than their brand-name equivalents, based on generic competition.
However, the generic version must contain the same active ingredient/s as the brand name. Also, generic drugs must not resemble any other drugs sold in the US, due to trademark laws; the flavor, color, shape, and any additional inactive ingredients must also be unique.
Are Generic Medications Safe to Use?
The FDA states that generic medications must be as effective as their brand-name counterparts, therefore having the same risks and benefits. They have issued certain strict requirements for a generic drug being the same as the brand-name drug in terms of:
- Safety
- Strength
- Dosage
- Mode of action
- Method of using
- Method of ingestion
- Ailment it treats
To further ensure safety, quality and effectiveness of the drugs, the FDA, including a detailed scientific analysis on the drug’s performance and ingredients, puts all generic drugs through a review process. Moreover, manufacturing plants producing generic drugs must maintain the same superior standards as those of a brand-name drug production unit – about 3,500 on-site inspections are conducted every year.
Another interesting aspect is that almost half of all generic drugs are being produced by brand-name companies themselves. They simply produce copies of their own drugs, or another company’s brand-name drugs, and sell them under name of the generic API.
Why is the Cost of Manufacturing Drugs in the US Much Higher than Other Countries?
For starters, in the US, negotiations with respect to drug prices occur between manufacturers and individual insurers – there is no involvement of the government. Insurers have developed a way for consumers to have choice and save money. While expensive drugs can still be covered, drugs in stage one have much lesser copay costs as compared to drugs in higher stages of development and usage.
When consumers discover that they’re paying $100 for a level two drug instead of $20 for a level one option, they ask their physician for a cheaper alternative. Manufacturers with a higher tiered drug make use of this opportunity and respond by crafting prescription coupons rather than lowering the general cost of the drug. So when consumers visit the pharmacy, they pay $20 for the drug, and the manufacturer pays the remaining $80 of their copay.
Secondly, drug companies in the US can reach out to users and sponsor drugs directly, without the need for advertising. This isn’t allowed in other countries. This creates demand and helps companies acquire hundreds of millions of dollars in advertising, which they recover by charging highly for the drugs.
While this system might reduce the out-of-pocket burden on consumers, manufacturers shift the overall costs back on insurers by simply increasing the price. These extra costs are then adjusted into the higher premiums charged to consumers.
How are Generic APIs Developed?
When it comes to development, API manufacturersmust firstly consider how to make the chemical compound (raw material) which will eventually become an API. Factors affecting the quality of the API, such as the degree of concentration and optimum temperature, are also considered. Once the basics are finalized, a series of preliminary experiments are performed to determine exactly how to make the raw material. Once developed, the latter undergoes a series of processes and intermediates to ultimately manufacture a large quantity of APIs. Quality control experiments are then conducts a testing laboratory to determine whether the API is ultrapure. After testing, quality assurance staff assesses the credibility of the entire manufacturing process, and confirms whether it is in accordance with FDA guidelines.
What Are Generic API Development Or Manufacturing Requirements?
As highlighted in the development section, the pharmaceutical industry is changing rapidly. Companies are no longer involved in handling every single step of the drug-manufacturing process, and nor is a single company involved in creating the API, building the capsule, and packaging the medicine.
As a means of ensuring public and patient safety, relevant governing institutions have established stringent screenings to ascertain quality and prevent defects in medications. Violating any of these instituted standards may result in fines or extremely expensive recalls for the pharmaceutical companies behind the manufacturers.
Manufacturers of generic APIs must adhere to certain standards which will help ascertain how potent the API in each drug is. These standards may vary significantly from brand to brand, for instance in terms of the test methods they use, resulting in various potencies. However, in all cases, manufacturers must verify the potency of their drugs via real-life patients and under laboratory conditions, as required by the FDA.
A very important aspect that API manufacturers must consider is the quality of APIs, since this has a substantial effect on the efficacy and safety of medications. Below par or compromised APIs can lead to serious health issues, resulting in chronic illnesses and even death.
Even when outsourced, APIs must adhere tothe strict regulations and guidelinesof the country they are dispatched to; overseas API manufacturing plants are still inspected by the US FDA.
What Is The Need For A Generics Class Implementation?
A generics class implementation system will help clarify the quality-by-design approach to regulatory authorities, signifying how the manufacturing process has been formulated while keeping in check quality criteria and other essentialattributes pertaining to the active ingredient.
How Do You Ensure Smooth Technology Transfer In API Manufacturing?
Technology transfer can be a laborious and complex process that requires teamwork, effective communication, and the utmost ability to foresee and resolve any possible obstacles that might arise during the process. Usually, a chemical process is transferred either to a different company with their own specific processes and protocols, or it can be the transfer of a project from an overseas Contract Manufacturing Organization (CMO) which, again, will have their own regulations and processes.
Effective and efficient transfer of technology takes place only when strict protocols are developed to share the knowledge and capabilities of a particular API manufacturing process.These foundations also lay the groundwork for the next stages of development, and allow Contract Development and Manufacturing Organizations (CDMOs) to allocate resources and effectively complete their work.
Hence, manufacturers of APIs must have a reliable technology transfer protocol to ensure no critical information is lost and no time is wasted when transferring technology to another firm or site. This guarantees a smooth process and helps attain the confidence of the sponsoring firm and the contractors associated with the transfer.
Certain key considerations to ensure an effective technology transfer are as follows:
- Sponsors must transparently share batch records, and any pertinent information related to obstacles and challenges.
- Manufacturing engineers must be directly involved with technical managers to oversee optimization, scale-up processes, and other critical steps.
- Chemists and GMO specialists must be on the same page regarding the use of raw materials, ensuring appropriate analytics and master batch records are compiled.
- Research and development chemists and GMP specialists must work together to get the scale-up process running, and must work with engineers to ensure the appropriate equipment and other instruments are available.
Market Trends in Generic APIs
Which Country is the Largest Producer of Generic Drugs?
Even though many pharmaceutical companies are centered in the US and UK, most API manufacturers are based overseas, including Asia, and particularly China and India.
As a means of cutting back on costs concerning equipment, infrastructure and employees, many companies are opting to outsource their manufacturing. The most prominent example is that of AstraZeneca Pharmaceuticals which operated several manufacturing units in the US. Now, only 15% of their APIs are produced within the country, and they also plan to finish this small percentage by outsourcing all of their manufacturing overseas.
While this does help produce drugs at an economical scale, a significant concern about the quality and safety of these APIs exists.
Which Is The Biggest Generic Drug Company?
The chief manufacturer of APIs is TEVA Pharmaceuticals, with the drug industry’s largest portfolio featuring more than 3,500 API products, making it the largest pharmaceutical company worldwide.
Established in 1901, TEVA has been providing healthcare workers, patients and caregivers accessible generic and original products. With generic sales amounting to $ 9 billion, almost 200 million individuals in over 60 countries rely on the company’s superior quality medicines on a daily basis.
TEVA Pharmaceuticals invests heavily in research and development (R&D) of generic drugs and biopharmaceuticals, relying on their legacy of over a century of formulating innovative methods to facilitate patients and improve their quality of life.
Top Suppliers of Generic APIs
Other industry leaders of generic APIs include:
- Sun Pharmaceutical Industries Ltd: Generic Sales $ 4 Billion
- Pfizer: Generic Sales $ 3.5 Billion
- Novartis International: Generic Sales $ 8.6 Billion
- Fresenius Medical Care: Generic Sales $ 3.2 Billion
- Aurobindo Pharma: Generic Sales $ 2.3 Billion
- Aspen Pharma: Generic Sales $ 2 Billion
- Lupin: Generic Sales $ 2.2 Billion
- Amneal Pharmaceuticals, Inc: Generic Sales $ 1.8 Billion
Each of these manufacturers specializes in diverse APIs, while some also offer generic products.
References
https://www.verywellhealth.com/api-active-pharmaceutical-ingredient-2663020
https://www.verywellhealth.com/generic-drug-safety-and-effectiveness-1738890
https://www.katsura-chemical.co.jp/en/drugs/
https://theconversation.com/why-cheaper-drugs-from-canada-likely-wont-cure-what-ails-us-121723
References
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
-
Webinar Leveraging Real-Time Clinical Data to Deliver Certainty in Solubility Enhancement and Modified Release Development
-
12th December 2024
-
3pm BST/ 4pm CET
-
-
Webinar The CPHI Sustainability Collective: A New Initiative to Support a Sustainable Pharma Value Chain
-
10th December 2024
-
3pm BST /4pm CET
-
-
Webinar Key to Success: A CDMO's Pathway to Biologics Excellence
-
5th November 2024
-
3pm BST/ 4pm CET
-
-
Webinar The Changing Dynamics of Global API Manufacturing
-
17th September 2024
-
3pm BST/ 4pm CET
-
-
Webinar Shaping the Future of Italy’s Pharma Market: Trends and Opportunities
-
4th September 2024
-
4pm CET/ 10am EST
-
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
-
Webinar The Next Frontier – Emerging Opportunities in the LATAM Pharma Market
-
21st November 2023
-
4pm CET / 10am EST
-
-
Webinar Vistamaxx™ MED - imagine the possibilities for healthcare product performance
-
10th October, 2023
-
4pm CET / 10am EST
-
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance







-comp246169.jpg)

















































































































.png)
.png)

.png)


.png)



















































































































.png)







.png)

.png)



.jpg)


