7
May
2024

Metrochem API Privated Limited
Exhibitor at CPHI North America 2024 stand 1313
About Us
Categories

-
IN
-
2015On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
Primary activities
Event information
CPHI North America 2024
-
07 May 2024 - 09 May 2024
-
Pennsylvania Convention Center, Philadelphia
-
Visit us at stand 1313
Products Featured at CPHI North America 2024
-
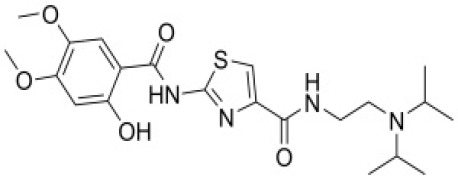
Product Acotiamide Hydrochloride
Gastroprokinetic - Commercial -
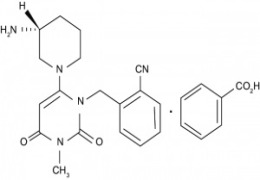
Product Alogliptin Benzoate
Antidiabetic - Commercial -
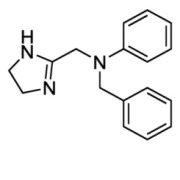
Product Antazoline Hydrochloride
Antihistamine - Commercial -
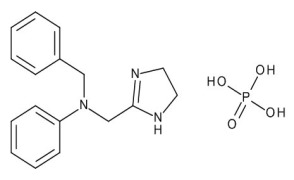
Product Antazoline Phosphate
Antihistamine - Commercial -
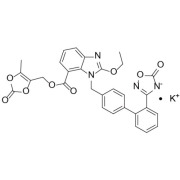
Product Azilsartan Medoxomil Potassium
Antihypertensives - Commercial
Metrochem API Private Limited Resources (1)
-
Brochure Metrochem API Private Limited API Brochure Q1 Fy22-23
Metrochem API Pvt ltd is one of the few vertically integrated Indian Pharmaceutical manufacturer approved by USFDA (EIR), WHO-GMP, COEFPRIS, KFDA & ISO 9001:2008 certification. We hold few process patents .Our products include API`s, Pellets and intermediates,CRAMS and P2P in wide variety of segments, mainly Anti Ulcerants, Anti allergic, Anti coagulants, Anti diabetic & Anti Hypertensive’s. Metrochem API Vizag`s manufacturing unit is a green field manufacturing facility in 50,000 sqmts with 700kl reaction volume. We have capability to take a kilo to multi ton batches in the new facility. This USFDA and EUGMP approvable facility started operations in May-2015
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance